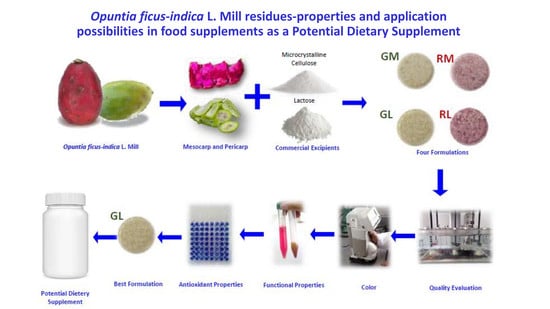
Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
Tablet Formulation
2.2. Quality Parameters
2.2.1. Mass Uniformity, Thickness, and Diameter
2.2.2. Tablet Breaking Force
2.2.3. Friability
2.2.4. Disintegration
2.3. Scanning Electron Microscopy (SEM)
2.4. Functional Properties: Lipid and Water Holding Capacity
2.5. Color Measurement
2.6. Antioxidant Properties
2.6.1. Extraction of Antioxidants
2.6.2. Total Phenolic Content (TPC)
2.6.3. Antioxidant Capacity by ABTS•+
2.6.4. Antioxidant Activity by DPPH•
2.6.5. Ferric Reducing/Antioxidant Power (FRAP)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quality Parameters
3.2. Scanning Electron Microscopy (SEM)
3.3. Functional Properties: Lipid and Water Holding Capacity
3.4. Color
3.5. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Strengths and Limitations
References
- Block, G.; Jensen, C.; Norkus, E.; Dalvi, T.; Wong, L.; McManus, J. Usage patterns, health, and nutritional status of long-term multiple dietary supplement users: A cross-sectional study. Nutr. J. 2008, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radimer, K.; Bindewald, B.; Hughes, J.; Ervin, B.; Swanson, C.; Picciano, M.F. Dietary supplement use by US adults: Data from the national health and nutrition examination survey, 1999–2000. Am. J. Epidemiol. 2004, 160, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food & Drug Administration. Dietary Supplements, 2019. U.S. Food & Drug Administration Website. Available online: https://www.fda.gov/consumers/consumer-updates/dietary-supplements (accessed on 21 April 2020).
- Da Costa, A.G.; Nunes, M.A.; Almeida, I.; Carvalho, M.; Barroso, M.F.; Alves, R.C.; Oliveira, M.B.P.P. Teas, dietary supplements and fruit juices: A comparative study regarding antioxidant activity and bioactive compounds. LWT 2012, 49, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, M.D.; Tomasello, B.; Giardina, R.C.; Dattilo, S.; Mazzei, V.; Sinatra, F.; Caruso, M.; D’Antona, N.; Renis, M. Sugar and mineral enriched fraction from olive mill wastewater for promising cosmeceutical application: Characterization, in vitroandin vivostudies. Food Funct. 2017, 8, 4713–4722. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant food residues as a source of nutraceuticals and functional foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [Green Version]
- Gurrieri, S.; Miceli, L.; Lanza, C.M.; Tomaselli, F.; Bonomo, R.P.; Rizzarelli, E. Chemical characterization of sicilian prickly pear (Opuntia ficus indica) and perspectives for the storage of its juice. J. Agric. Food Chem. 2000, 48, 5424–5431. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Avellaneda, Z.J.E.; Martín-Belloso, O.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; García-García, R.; Torres, J.A.; Welti-Chanes, J. Effect of high hydrostatic pressure on the content of phytochemical compounds and antioxidant activity of prickly pears (opuntia ficus-indica) beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Stintzing, F.C. Cactus pear fruits (Opuntia spp.): A review of processing technologies and current uses. J. Prof. Assoc. Cactus. 2006, 8, 1–25. [Google Scholar]
- Ramírez-Moreno, E.; Hervert-Hernández, D.; Sánchez-Mata, M.C.; Díez-Marqués, C.; Goñi, I. Intestinal bioaccessibility of polyphenols and antioxidant capacity of pulp and seeds of cactus pear. Int. J. Food Sci. Nutr. 2011, 62, 839–843. [Google Scholar] [CrossRef]
- Galati, E.; Tripodo, M.; Trovato, A.; Miceli, N.; Monforte, M. Biological effect of Opuntia ficus indica (L.) Mill. (Cactaceae) waste matter. J. Ethnopharmacol. 2002, 79, 17–21. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Kovačević, D.B.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M. Impact of conventional and nonconventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El-Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El-Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M.; et al. Nopal cactus (opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compost. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- El-Kossori, R.L.; Villaume, C.; El-Boustani, E.; Sauvaire, Y.; Mejean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus-indica sp.). Plant Food Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Carranza, P.; Jattar-Santiago, K.Y.; Avila-Sosa, R.; Pérez-Xochipa, I.; Guerrero-Beltrán, J.A.; Ochoa-Velasco, C.E.; Ruiz-López, I. Antioxidant fortification of yogurt with red cactus pear peel and its mucilage. CyTA-J. Food 2019, 17, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Namir, M.; Elzahar, K.; Ramadan, M.F.; and Allaf, K. Cactus pear peel snacks prepared by instant pressure drop texturing: Effect of process variables on bioactive compounds and functional properties. J. Food Meas. Charact. 2017, 11, 388–400. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chow, C.J.; Fang, Y.J. Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J. Food Drug Anal. 2011, 19, 318–323. [Google Scholar]
- Bansal, J.; Malviya, R.; Malaviya, T.; Bhardwaj, V.; Sharma, P. Evaluation of Banana peel Pectin as excipient in solid Oral Dosage Form. Glob. J. Pharm. 2014, 8, 275–278. [Google Scholar]
- Srivastava, P.; Malviya, R. Extraction, characterization and evaluation of orange peel waste derived pectin as a pharmaceutical excipient. Nat. Prod. J. 2011, 1, 65–70. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N.; Reshetnikov, A.; Kasatkin, A.; Kopylov, M.; Baimurzin, D. About what is happening in the stomach after swallowing human river pebbles, gravel, chalk, clay and tablets drugs. Építöanyag (Online). Epitoanyag-J. Silic. Based Compos. Mater. 2016, 4, 110. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Sun, C.C. Validation and applications of an expedited tablet friability method. Int. J. Pharm. 2009, 484, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Normas. Non industrialized food products for human use, fresh fruit Cactus pear (Opuntia spp.) Specifications. Available online: https://www.colpos.mx/bancodenormas/nmexicanas/NMX-FF-030-1995.PDF (accessed on 13 March 2020).
- Uniformity of Dosage Units (USP 911), 2011. Pharmacopeia US. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q0304_stage_6_monograph_25_feb_2011.pdf (accessed on 13 January 2019).
- Tablet Breaking Force (USP 1217), 2016. Pharmacopeia US. Available online: https://www.in-pharmatechnologist.com/Library/USP-1217-Tablet-Breaking-Force-A-New-Chapter-for-Tablet-Hardness-Testing (accessed on 13 January 2019).
- Tablet Friability (USP 1216), 2016. Pharmacopeia US. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/g06_pf_ira_32_2_2006.pdf (accessed on 13 January 2019).
- Disintegration (USP 701), 2019. Pharmacopeia US. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/april-2019-m99460.pdf (accessed on 13 January 2019).
- Valencia, F.; Román, M. Physicalchemical and functional characterization of three commercial concentrates from dietary fiber. Vitae 2006, 13, 54–60. [Google Scholar]
- Pathare, P.B.; Umezuruike, L.O.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, J.J.; De-La-Garza, T.H.; Zugasti, A.; Ruth, C.; Noé, A.C.; Heliodoro, D.L.G.T.; Jasso, R.M.R. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac. J. Trop. Biomed. 2013, 6, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanís-García, E.; Añorve-Morga, J.; Quintero-Lira, A. Organic acids, antioxidants, and dietary fiber of mexican blackberry (rubus fruticosus) residues cv. Tupy. J. Food Qual. 2018, 6, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Seville, J.P. A comparative study of compaction properties of binary and bilayer tablets. Powder Technol. 2009, 189, 285–294. [Google Scholar] [CrossRef]
- Awofisayo, S.O.; Awofisayo, O.A.; Eyen, N.; Udoh, I.E. Comparative assessment of the quality control measurements of multisource ofloxacin tablets marketed in Nigeria. Dissolution Technol. 2010, 17, 20–25. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression physics in the formulation development of tablets. Crit. Rev. Ther. Drug Carr. Syst. 2006, 23, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Khule, N.R.; Mahale, N.B.; Shelar, D.S.; Rokade, M.M.; Chaudhari, S.R. Extraction of pectin from citrus fruit peel and use as natural binder in paracetamol tablet. Der. Pharm. Lett. 2012, 4, 558–564. [Google Scholar]
- US Department of Health and Human Services & F. and DA. Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules Guidance for Industry, 2015. U.S. Food & Drug Administration Website. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.html (accessed on 13 March 2020).
- Divate, S.; Kavitha, K.; Sockan, G.N. Fast disintegrating tablets—An emerging trend. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 2. [Google Scholar]
- Patel, S.; Sun, C.C. Macroindentation hardness measurement modernization and applications. Int. J. Pharm. 2016, 506, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Sackett, G.; Liu, L. Development, optimization, and scale-up of process parameters: Pan coating. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2017; pp. 953–996. [Google Scholar]
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar]
- Quodbach, J.; Kleinebudde, P. Performance of tablet disintegrants: Impact of storage conditions and relative tablet density. Pharm. Dev. Technol. 2015, 6, 762–768. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Iqbal, J.; Shirazian, S.; Walker, G.M.; Collins, M.N. Effect of lignin on the release rate of acetylsalicylic acid tablets. Int. J. Biol. Macromol. 2019, 124, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Van Den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today: Technol. 2012, 9, 79–85. [Google Scholar] [CrossRef]
- Filson, P.B. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 7, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Boschini, F.; Delaval, V.; Traina, K.; Vandewalle, N. Linking flowability and granulometry of lactose powders. Int. J. Pharm. 2015, 494, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte-Robles, L. The excipients and their functionality in pharmaceutical solid products. Rev. Mex. Cienc. Farm. 2011, 42, 18–36. [Google Scholar] [CrossRef]
- Kaerger, J.S.; Edge, S.; Price, R. Influence of particle size and shape on flowability and compactibility of binary mixtures of paracetamol and microcrystalline cellulose. Eur. J. Pharm. Sci. 2004, 22, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-products of Opuntia ficus-indica as a source of antioxidant dietary fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S. Handbook of Dietary Fiber; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Cardenas, A.; Higuera-Ciapara, I.; Goycoolea, F. Rheology and aggregation of cactus (Opuntia ficus indica) mucilage in solution. J. Prof. Assoc. Cactus Dev. 1997, 2, 152–159. [Google Scholar]
- Cardador-Martínez, A.; Jiménez-Martínez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Food Sci. Technol. 2011, 31, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Chamorro, R.A.M.; Mamani, E.C. Importancia de la fibra dietética, sus propiedades funcionales en la alimentación humana y en la industria alimentaria. Rev. Investing Cienc. Tecnol. Aliment. 2010, 1, 4–17. [Google Scholar]
- Lario, Y.E.; Sendra, E.; Garcıa-Pérez, J.; Fuentes, C.; Sayas-Barberá, E.; Fernández-López, J. Preparation of high dietary fiber powder from lemon juice by-products. Innov. Food Sci. Emerg. 2004, 5, 113–117. [Google Scholar] [CrossRef]
- Caprita, A.; Caprita, R.; Simulescu, V.O.; Drehe, R.M. Dietary fiber: Chemical and functional properties. J. Agroaliment Process Technol. 2010, 16, 406–410. [Google Scholar]
- McLellan, M.R.; Lind, L.R. Hue angle determinations and statistical analysis for multiquadrant Hunter, L., a, b data. J. Food Qual. 1995, 3, 235–240. [Google Scholar] [CrossRef]
- Sánchez, F.D.; López, E.M.S.; Kerstupp, S.F.; Ibarra, R.V.; Scheinvar, L. Colorant extraction from red prickly pear (Opuntia lasiacantha) for food application. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1330–1337. [Google Scholar]
- Cota-Sánchez, J.H. Nutritional composition of the prickly pear (Opuntia ficus-indica) fruit. In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2016; pp. 691–712. [Google Scholar]
- Cejudo-Bastante, M.J.; Chaalal, M.; Louaileche, H.; Parrado, H.J.; Heredia, F.J. Betalain profile, phenolic content, and color characterization of different parts and varieties of Opuntia ficus-indica. J. Agric. Food Chem. 2014, 62, 8491–8499. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.D.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Montañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Filardo-Kerstupp, T. Antioxidant and Antimicrobial Properties of Cactus Pear (Opuntia) Seed Oils. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tokuşoğlu, Ö. Innovative mandarin peel effervescent tablet as antioxidant and anticarcinogen food supplement: Bioactive flavanones and phenolic acids by HPLC-DAD and LC-esiqtoff mass spectrometry. Food Health Technol. Innov. 2018, 1, 75–80. [Google Scholar]
- Seifried, R.M.; Harrison, E.; Seifried, H.E. Antioxidants in health and disease. Nutr. Prev. Treat. Dis. 2017, 321, 346. [Google Scholar]
- Aguilera, C.A.; Tovar, J.C.; Rosales, S.A.; Pérez, L.; Guadalupaseso, M.; Arroyo, H.S.; Hernández, M.S. Límites máximos de residuos e intervalos de seguridad de plaguicidas en tuna, Opuntia ficus-indica. Master’s Thesis, Colegio de Postgraduados (COLPOS) Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico City, Mexico, 2008. [Google Scholar]
- Mottese, A.F.; Naccari, C.; Vadalà, R.; Bua, G.D.; Bartolomeo, G.; Rando, R.; Giacomo, D.; Nicola, C. Traceability of Opuntia ficus-indica L. Miller by ICP-MS multi-element profile and chemometric approach. J. Sci. Food Agric. 2018, 98, 198–204. [Google Scholar] [CrossRef]


| GM | GL | RM | RL | |
|---|---|---|---|---|
| Weight (g) | 0.7 ± 0 d | 0.9 ± 0 b | 0.8 ± 0 c | 1 ± 0 a |
| Thickness (mm) | 4.6 ± 0 c | 4.8 ± 0 b | 4.6 ± 0 c | 5.6 ± 0 a |
| Diameter (mm) | 14 ± 0 a | 14 ±.0 a | 14 ± 0 a | 14 ± 0 a |
| Breaking force (N) | 121 ± 8 b | 162 ± 29 a | 164 ± 29 a | 96 ± 13 b |
| Friability (%) | 0.3 ± 0 a | 0.1 ± 0 b | 0.3 ± 0 a | 0.1 ± 0 b |
| Disintegration (min) | 2 ± 0 c | 66 ± 2 a | 19 ± 1 b | 67.3 ± 4 a |
| LHC (g/g) | 5.3 ± 0 a | 4.5 ± 0 b | 4.5 ± 0 b | 3.8 ± 0 c |
| WHC (g/g) | 5.5 ± 0 a | 4.2 ± 0 b | 4.6 ± 0 b | 3.6 ± 0 c |
| GM | GL | RM | RL | |
|---|---|---|---|---|
| L* | 49 ± 0 a | 48 ± 1 a | 40 ± 1 b | 41 ± 0 b |
| a* | −2 ± 0 c | −2 ± 0 c | 7 ± 0 b | 9 ± 0 a |
| b* | 16 ± 0 a | 15 ± 0 a | 4 ± 0 b | 4 ± 0 b |
| h° | 97 ± 0 a | 98 ± 0 a | 31 ± 1 b | 25 ± 0 c |
| C* | 16 ± 0 a | 15 ± 0 a | 9 ± 0 c | 11 ± 0 b |
| GM | GL | RM | RL | |
|---|---|---|---|---|
| TPC (mg GAE/100 g) | 718 ± 11 b | 734 ± 8 a | 649 ± 12 c | 651 ± 8 c |
| DPPH (% Inhibition) | 20 ± 1 b | 20 ± 2 b | 24 ± 2 a | 24 ± 1 a |
| ABTS (µmol TE/100 g) | 4628 ± 412 a | 4637 ± 242 a | 4365 ± 392 a | 4610 ± 326 a |
| FRAP (µmol Fe2/100 g) | 17 ± 0 b | 16 ± 1 c | 19 ± 0 a | 17 ± 0 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzur-Valdespino, S.; Ramírez-Moreno, E.; Arias-Rico, J.; Jaramillo-Morales, O.A.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Córdoba-Díaz, M.; Córdoba-Díaz, D.; Cruz-Cansino, N.d.S. Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements. Appl. Sci. 2020, 10, 3260. https://doi.org/10.3390/app10093260
Manzur-Valdespino S, Ramírez-Moreno E, Arias-Rico J, Jaramillo-Morales OA, Calderón-Ramos ZG, Delgado-Olivares L, Córdoba-Díaz M, Córdoba-Díaz D, Cruz-Cansino NdS. Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements. Applied Sciences. 2020; 10(9):3260. https://doi.org/10.3390/app10093260
Chicago/Turabian StyleManzur-Valdespino, Salvador, Esther Ramírez-Moreno, José Arias-Rico, Osmar Antonio Jaramillo-Morales, Zuli Guadalupe Calderón-Ramos, Luis Delgado-Olivares, Manuel Córdoba-Díaz, Damián Córdoba-Díaz, and Nelly del Socorro Cruz-Cansino. 2020. "Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements" Applied Sciences 10, no. 9: 3260. https://doi.org/10.3390/app10093260