Future Climate Change Conditions May Compromise Metabolic Performance in Juveniles of the Mud Crab Scylla serrata
Abstract
:1. Introduction
2. Materials and Methods
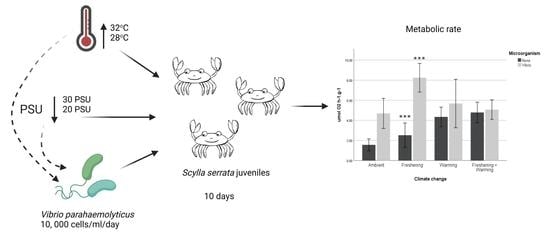
2.1. Experimental Design and Setup
2.2. Determination of Metabolic Rates
2.3. Data Analysis
3. Results
3.1. Survival Rates
3.2. Metabolic Rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1–30. [Google Scholar] [CrossRef]
- Bryndum-Buchholz, A.; Tittensor, D.P.; Blanchard, J.L.; Cheung, W.W.L.; Coll, M.; Galbraith, E.D.; Jennings, S.; Maury, O.; Lotze, H.K. Twenty-first-century climate change impacts on marine animal biomass and ecosystem structure across ocean basins. Glob. Chang. Biol. 2019, 25, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Balaguru, K.; Foltz, G.R.; Leung, L.R.; Emanuel, K.A. Global warming-induced upper-ocean freshening and the intensification of super typhoons. Nat. Commun. 2016, 7, 13670. [Google Scholar] [CrossRef] [Green Version]
- Keeling, R.E.; Kortzinger, A.; Gruber, N. Ocean deoxygenation in a warming world. Ann. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [Green Version]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, L.; Comte, A.; Langdon, C.; Ekstrom, J.A.; Cooley, S.R.; Suatoni, L.; Beck, M.W.; Brander, L.M.; Burke, L.; Cinner, J.E.; et al. Coral Reefs and People in a High-CO2 World: Where Can Science Make a Difference to People? PLoS ONE 2016, 11, e0164699. [Google Scholar] [CrossRef]
- Wu, H.C.; Dissard, D.; Douville, E.; Blamart, D.; Bordier, L.; Tribollet, A.; Le Cornec, F.; Pons-Branchu, E.; Dapoigny, A.; Lazareth, C.E. Surface ocean pH variations since 1689 CE and recent ocean acidification in the tropical South Pacific. Nat. Commun. 2018, 9, 2543. [Google Scholar] [CrossRef] [Green Version]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate Change and Water; Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008. [Google Scholar]
- Jennerjahn, T.C. Biogeochemical response of tropical coastal systems topresent and past environmental change. Earth Sci. Rev. 2012, 114, 19–41. [Google Scholar] [CrossRef]
- Asch, R.G.; Cheung, W.W.L.; Reygondeau, G. Future marine ecosystem drivers, biodiversity, and fisheries maximum catch potential in Pacific Island countries and territories under climate change. Mar. Policy 2018, 88, 285–294. [Google Scholar] [CrossRef]
- Lotze, H.K.; Tittensor, D.P.; Bryndum-Buchholz, A.; Eddy, T.D.; Cheung, W.W.L.; Galbraith, E.D.; Barange, M.; Barrier, N.; Bianchi, D.; Blanchard, J.L.; et al. Global ensemble projections reveal trophic amplification of ocean biomass declines with climate change. Proc. Natl. Acad. Sci. USA 2019, 116, 12907–12912. [Google Scholar] [CrossRef] [Green Version]
- Burge, C.A.; Mark Eakin, C.; Friedman, C.S.; Froelich, B.; Hershberger, P.K.; Hofmann, E.E.; Petes, L.E.; Prager, K.C.; Weil, E.; Willis, B.L.; et al. Climate change influences on marine infectious diseases: Implications for management and society. Ann. Rev. Mar. Sci. 2014, 6, 249–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godhe, A.; Narayanaswamy, C.; Klais, R.; Moorthy, K.S.V.; Ramesh, R.; Rai, A.; Reddy, H.R.V. Long-term patterns of net phytoplankton and hydrography in coastal SE Arabian Sea: What can be inferred from genus level data? Estuar. Coast. Shelf Sci. 2015, 162, 69–75. [Google Scholar] [CrossRef]
- Turner, L.M.; Alsterberg, C.; Turner, A.D.; Girisha, S.K.; Rai, A.; Havenhand, J.N.; Venugopal, M.N.; Karunasagar, I.; Godhe, A. Pathogenic marine microbes influence the effects of climate change on a commercially important tropical bivalve. Sci. Rep. 2016, 6, 32413. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.M.; Havenhand, J.N.; Alsterberg, C.; Turner, A.D.; Rai, A.; Venugopal, M.N.; Karunasagar, I.; Godhe, A. Toxic Algae Silence Physiological Responses to Multiple Climate Drivers in a Tropical Marine Food Chain. Front. Physiol. 2019, 10, 373. [Google Scholar] [CrossRef] [Green Version]
- Vezzulli, L.; Pezzati, E.; Brettar, I.; Hofle, M.; Pruzzo, C. Effects of Global Warming on Vibrio Ecology. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Urtaza, J.; Bowers, J.C.; Trinanes, J.; DePaola, A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res. Int. 2010, 43, 1780–1790. [Google Scholar] [CrossRef]
- Vezzulli, L.; Brettar, I.; Pezzati, E.; Reid, P.C.; Colwell, R.R.; Hofle, M.G.; Pruzzo, C. Long-term effects of ocean warming on the prokaryotic community: Evidence from the vibrios. ISME J. 2012, 6, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Vianna, B.D.S.; Miyai, C.A.; Augusto, A.; Costa, T.M. Effects of temperature increase on the physiology and behavior of fiddler crabs. Physiol. Behav. 2020, 215, 112765. [Google Scholar] [CrossRef]
- Giomi, F.; Raicevich, S.; Giovanardi, O.; Pranovi, F.; Muro, P.D.; Beltramini, M. Catch me in winter! Seasonal variation in air temperature severely enhances physiological stress and mortality of species subjected to sorting operations and discarded during annual fishing activities. Hydrobiologia 2008, 606, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Matozzo, V.; Gallo, C.; Marin, M.G. Effects of temperature on cellular and biochemical parameters in the crab Carcinus aestuarii (Crustacea, Decapoda). Mar. Environ. Res. 2011, 71, 351. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, E.; Dugan, J.E.; Hubbard, D.M.; Contreras, H.; Duarte, C.; Acuna, E.; Schoeman, D.S. Macroscale patterns in body size of intertidal crustaceans provide insights on climate change effects. PLoS ONE 2017, 12, e0177116. [Google Scholar] [CrossRef] [Green Version]
- Hews, S.; Allen, Z.; Baxter, A.; Rich, J.; Sheikh, Z.; Taylor, K.; Wu, J.; Zakoul, H.; Brodie, R. Field-based body temperatures reveal behavioral thermoregulation strategies of the Atlantic marsh fiddler crab Minuca pugnax. PLoS ONE 2021, 16, e0244458. [Google Scholar] [CrossRef]
- McGaw, I.J. Behavioral Thermoregulation in Hemigrapsus nudus, the Amphibious Purple Shore Crab. Biol. Bull. 2003, 204, 38–49. [Google Scholar] [CrossRef]
- Madeira, C.; Leal, M.C.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal stress and energy metabolism in two circumtropical decapod crustaceans: Responses to acute temperature events. Mar. Environ. Res. 2018, 141, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D. Climate change enhances disease processes in crustaceans: Case studies in lobsters, crabs, and shrimps. J. Crustacean Biol. 2019, 39, 673–683. [Google Scholar] [CrossRef]
- Waller, J.D.; Wahle, R.A.; McVeigh, H.; Fields, D.M.; Juanes, F. Linking rising pCO2 and temperature to the larval development and physiology of the American lobster (Homarus americanus). ICES J. Mar. Sci. 2017, 74, 1210–1219. [Google Scholar] [CrossRef]
- Gravinese, P.M.; Enochs, I.C.; Manzello, D.P.; van Woesik, R. Warming and pCO2 effects on Florida stone crab larvae. Estuar. Coast. Shelf Sci. 2018, 204, 193–201. [Google Scholar] [CrossRef]
- Pansch, C.; Nasrolahi, A.; Appelhans, Y.S.; Wahl, M. Impacts of ocean warming and acidification on the larval development of the barnacle Amphibalanus improvisus. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 48–55. [Google Scholar] [CrossRef]
- Turner, L.M.; Ricevuto, E.; Massa Gallucci, A.; Lorenti, M.; Gambi, M.C.; Calosi, P. Metabolic responses to high pCO2 conditions at a CO2 vent site in juveniles of a marine isopod species assemblage. Mar. Biol. 2016, 163, 211. [Google Scholar] [CrossRef] [Green Version]
- Small, D.P.; Calosi, P.; Boothroyd, D.; Widdicombe, S.; Spicer, J.I. Stage-Specific Changes in Physiological and Life-History Responses to Elevated Temperature and PCO2 during the Larval Development of the European Lobster Homarus gammarus (L.). Physiol. Biochem. Zool. 2015, 88, 494–507. [Google Scholar] [CrossRef]
- Walther, K.; Anger, K.; Pörtner, H.O. Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54° vs. 79° N). Mar. Ecol. Prog. Ser. 2010, 417, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res. 2012, 76, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Small, D.P. The Effects of Elevated Temperature and pCO2 on Thedevelopmental Eco-Physiology of the European Lobster, Homarus gammarus (L.). Ph.D. Thesis, Plymouth University, Plymouth, UK, 2013. [Google Scholar]
- Pörtner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential persistence in a changing ocean. Oceanogr. Mar. Biol. 2011, 49, 1–42. [Google Scholar]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, S.U.; Nagelkerken, I.; Ferreira, C.M.; Ullah, H.; Connell, S.D. Boosted food web productivity through ocean acidification collapses under warming. Glob. Chang. Biol. 2017, 23, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Bartomeus, I.; Saavedra, S.; Rohr, R.P.; Godoy, O. Experimental evidence of the importance of multitrophic structure for species persistence. Proc. Natl. Acad. Sci. USA 2021, 118, e2023872118. [Google Scholar] [CrossRef]
- Le Vay, L. Ecology and Management of Mud Crab Scylla spp. Asian Fish. Sci. 2001, 14, 101–111. [Google Scholar] [CrossRef]
- Mirera, O.D. Trends in exploitation, development and management of artisanal mud crab (Scylla serrata-Forsskal-1775) fishery and small-scale culture in Kenya: An overview. Ocean Coast. Manag. 2011, 54, 844–855. [Google Scholar] [CrossRef]
- Sen, S.; Homechaudhuri, S. Population characteristics and trends in artisanal fishery of Scylla serrata (Forsskål, 1775) in Indian Sundarban: Implications on future managements. Ocean Coast. Manag. 2017, 143, 105–114. [Google Scholar] [CrossRef]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): Current knowledge and future challenges. Hydrobiologia 2015, 763, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Flint, N.; Anastasi, A.; De Valck, J.; Chua, E.M.; Rose, A.K.; Jackson, E.L. Using mud crabs (Scyllaserrata) as environmental indicators in a harbour health report card, Australas. J. Environ. Manag. 2021, 28, 188–212. [Google Scholar] [CrossRef]
- Nandkeolyar, N.; Raman, M.; Kiran, G.S. Comparative Analysis of Sea Surface Temperature Pattern in the Eastern and Western Gulfs of Arabian Sea and the Red Sea in Recent Past Using Satellite Data. Int. J. Oceanogr. 2013, 2013, 501602. [Google Scholar] [CrossRef]
- Ruscoe, I.M.; Shelley, C.C.; Williams, G.R. The combined effects of temperature and salinity on growth and survival of juvenile mud crabs (Scylla serrata Forskål). Aquaculture 2004, 238, 239–247. [Google Scholar] [CrossRef]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquac. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Chen, H.; Liu, S.; Guo, Z.; Cheng, C.; Ma, H.; Feng, J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci. Rep. 2020, 10, 14329. [Google Scholar] [CrossRef]
- Hong To, T.T.; Yanagawa, H.; Khanh Thuan, N.; Hiep, D.M.; Cuong, D.V.; Khai, L.T.L.; Taniguchi, T.; Kubo, R.; Hayashidani, H. Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam. Biology 2020, 9, 312. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Das, S.C.; Kumar, A. Occurrence of pathogenic Vibrio parahaemolyticus in crustacean shellfishes in coastal parts of Eastern India. Vet. World 2016, 9, 330–336. [Google Scholar] [CrossRef] [Green Version]
- de Souza Valente, C.; Wan, A.H. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Xie, J.; Mei, H.; Jin, S.; Bu, L.; Wang, X.; Wang, C.; Zhao, Q.; Ma, R.; Zhou, S. Outbreak of vibriosis associated with Vibrio parahaemolyticus in the mud crab Scylla paramamosain cultured in China. Dis. Aquat. Org. 2021, 144, 187–196. [Google Scholar] [CrossRef]
- Su, Y.C.; Liu, C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Boenish, R.; Kritzer, J.P.; Kleisner, K.; Steneck, R.S.; Werner, K.M.; Zhu, W.; Schram, F.; Rader, D.; Cheung, W.; Ingles, J.; et al. The global rise of crustacean fisheries. Front. Ecol. Environ. 2022, 20, 102–110. [Google Scholar] [CrossRef]
- Fielder, D.; Allan, G. Executive summary and recommendations. In Mud crab aquaculture in Australia and Southeast Asia. In Proceedings of a Scoping Study and Workshop; Allan, G., Fielder, D., Eds.; ACIAR Working Paper No. 54; Australian Centre for International Agricultural Research: Canberra, Australia, 2004; pp. 61–62. [Google Scholar]
- Chandrapavan, A.; Caputi, N.; Kangas, M.I. The Decline and Recovery of a Crab Population from an Extreme Marine Heatwave and a Changing Climate. Front. Mar. Sci. 2019, 6, 510. [Google Scholar] [CrossRef]
- Hamasaki, K. Effects of temperature on the egg incubation period, survival and developmental period of larvae of the mud crab Scylla serrata (Forskål) (Brachyura: Portunidae) reared in the laboratory. Aquaculture 2003, 219, 561–572. [Google Scholar] [CrossRef]
- Baylon, J.C. Effects of Salinity and Temperature on Survival and Development of Larvae and Juveniles of the Mud Crab, Scylla serrata (Crustacea: Decapoda: Portunidae). J. World Aquac. Soc. 2010, 41, 858–873. [Google Scholar] [CrossRef]
- Mitchell, S.B.; Jennerjahn, T.C.; Vizzini, S.; Zhang, W. Changes to processes in estuaries and coastal waters due to intense multiple pressures–An introduction and synthesis. Estuar. Coast. Shelf Sci. 2015, 156, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.C.; Chia, P.G. Oxygen uptake and nitrogen excretion of juvenile Scylla serrata at different temperature and salinity levels. J. Crustacean Biol. 1996, 16, 437–442. [Google Scholar] [CrossRef]
- Romano, N.; Wu, X.; Zeng, C.; Genodepa, J.; Elliman, J. Growth, osmoregulatory responses and changes to the lipid and fatty acid composition of organs from the mud crab, Scylla serrata, over a broad salinity range. Mar. Biol. Res. 2014, 10, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Lucu, Č.; Towle, D.W. Na++K+-ATPase in gills of aquatic crustacea. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 195–214. [Google Scholar] [CrossRef]
- Boonsanit, P.; Pairohakul, S. Effects of salinity on haemolymph osmolality, gill Na+/K+ ATPase and antioxidant enzyme activities in the male mud crab Scylla olivacea (Herbst, 1796). Mar. Biol. Res. 2021, 17, 86–97. [Google Scholar] [CrossRef]
- Rahi, M.L.; Ferdusy, T.; Wali Ahmed, S.; Khan, M.N.; Aziz, D.; Salin, K.R. Impact of salinity changes on growth, oxygen consumption and expression pattern of selected candidate genes in the orange mud crab (Scylla olivacea). Aquac. Res. 2020, 51, 4290–4301. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D.; Schraga, T.S.; Nejad, E.; Martin, C. Ecosystem variability along the estuarine salinity gradient: Examples from long-term study of San Francisco Bay. Limnol. Oceanogr. 2017, 62, S272–S291. [Google Scholar] [CrossRef] [Green Version]
- Paital, B.; Chainy, G.B. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Chainy, G.B. Effects of temperature on complexes I and II mediated respiration, ROS generation and oxidative stress status in isolated gill mitochondria of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Wang, G.-Z.; Wu, L.-S.; Zeng, Z.-S.; Chen, X.-L. Seasonal change in mitochondrial function and metabolic enzyme activity of different populations of the mud crab, Scylla paramamosain, from China. Aquaculture 2013, 376–379, 68–75. [Google Scholar] [CrossRef]
- Vinagre, C.; Mendonca, V.; Cereja, R.; Abreu-Afonso, F.; Dias, M.; Mizrahi, D.; Flores, A.A.V. Ecological traps in shallow coastal waters-Potential effect of heat-waves in tropical and temperate organisms. PLoS ONE 2018, 13, e0192700. [Google Scholar] [CrossRef] [Green Version]
- Lozán, J.L. On the threat to the European crayfish: A contribution with the study of the activity behaviour of four crayfish species (Decapoda: Astacidae). Limnologica 2000, 30, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Cogo, G.B.; Biasi, C.; Severo, E.S.; Loro, V.; Santos, S. Environmental warming induces behavioral and metabolic changes in a freshwater crustacean−aeglids as a model organism. Ann. Limnol. 2018, 54, 7. [Google Scholar] [CrossRef]
- Sen Gupta, A.; Thomsen, M.; Benthuysen, J.A.; Hobday, A.J.; Oliver, E.; Alexander, L.V.; Burrows, M.T.; Donat, M.G.; Feng, M.; Holbrook, N.J.; et al. Drivers and impacts of the most extreme marine heatwave events. Sci. Rep. 2020, 10, 19359. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, G.; Sardanelli, F. Statistical significance: P value, 0.05 threshold, and applications to radiomics—Reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavilla-Pitogo, C.R.; de la Peña, L.D. Diseases in Farmed Mud Crabs Scylla spp.: Diagnosis, Prevention, and Control; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo, PH, USA, 2004. [Google Scholar]
- Jones, J.L. VIBRIO | Introduction, Including Vibrio parahaemolyticus, Vibrio vulnificus, and Other Vibrio Species. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 691–698. [Google Scholar] [CrossRef]
- Sullivan, T.J.; Neigel, J.E. Effects of temperature and salinity on prevalence and intensity of infection of blue crabs, Callinectes sapidus, by Vibrio cholerae, V. parahaemolyticus, and V. vulnificus in Louisiana. J. Invertebr. Pathol. 2018, 151, 82–90. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apine, E.; Mani, M.K.; Rai, P.; Karunasagar, I.; Turner, L.M. Future Climate Change Conditions May Compromise Metabolic Performance in Juveniles of the Mud Crab Scylla serrata. J. Mar. Sci. Eng. 2022, 10, 582. https://doi.org/10.3390/jmse10050582
Apine E, Mani MK, Rai P, Karunasagar I, Turner LM. Future Climate Change Conditions May Compromise Metabolic Performance in Juveniles of the Mud Crab Scylla serrata. Journal of Marine Science and Engineering. 2022; 10(5):582. https://doi.org/10.3390/jmse10050582
Chicago/Turabian StyleApine, Elina, Madhu K. Mani, Praveen Rai, Indrani Karunasagar, and Lucy M. Turner. 2022. "Future Climate Change Conditions May Compromise Metabolic Performance in Juveniles of the Mud Crab Scylla serrata" Journal of Marine Science and Engineering 10, no. 5: 582. https://doi.org/10.3390/jmse10050582