Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. CO2 Hydrogenation to Methanol and Methane
3.2. Thermodynamic Equilibrium
3.3. Kinetic Behavior in a Continuous Flow Catalytic Reactor
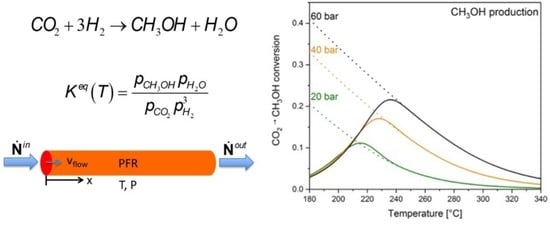
3.3.1. Plug Flow Reactor
3.3.2. Continuously Stirred Tank Reactor
- The volume of the reactor tank is given by Vtank;
- The reactants enter the tank with a flow velocity vflow through an inlet aperture, the area of which is Ainlet;
- The remaining variables have the same meaning as for the PFR described in Figure 6;
- The solution of the CSTR equations is self-consistent and requires the component production rates Rj to be evaluated at the exit of the reactor [41].
3.4. Modified Plug Flow Reactor
3.4.1. Looped Plug Flow Reactor with Recycling
3.4.2. Sorption-Enhanced Plug Flow Reactor
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tripodi, A.; Compagnoni, M.; Martinazzo, R.; Ramis, G.; Rossetti, I. Process simulation for the design and scale up of heterogeneous catalytic process: Kinetic modeling issues. Catalysis 2017, 7, 159. [Google Scholar] [CrossRef]
- Haydary, J. Chemical Process Design and Simulation: Aspen Plus and Aspen HYSYS Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Savelski, M.J.; Hesketh, R.P. Issues encountered with students using process simulators. Age 2002, 8, 1. [Google Scholar]
- Fogler, S.H. Elements of Chemical Reaction Engineering; Pearson Education Inc.: Upper Saddle River, NJ, USA, 1987. [Google Scholar]
- Davis, M.E.; Davis, R.J. Fundamentals of Chemical Reaction Engineering; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Manos, G. Introduction to Chemical Reaction Engineering. In Concepts of Chemical Engineering 4 Chemists; Simons, S., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hill, C.G.; Root, T.W. Introduction to Chemical Engineering Kinetics and Reactor Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Hagen, J. Industrial Catalysis; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Carvill, B.T.; Hufton, J.R.; Anand, M.; Sircar, S. Sorption-Enhanced Reaction Process. AIChE J. 1996, 42, 2765–2772. [Google Scholar] [CrossRef]
- Swaddle, T.W. Inorganic Chemistry—An Industrial and Environmental Perspective; Academic Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Graaf, G.H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Kinetics of low-pressure Methanol Synthesis. Chem. Eng. Sci. 1988, 43, 3185–3195. [Google Scholar] [CrossRef]
- Graaf, G.H.; Scholtens, H.; Stamhuis, E.J.; Beenackers, A.A.C.M. Intra-particle Diffusion Limitations in low-pressure Methanol Synthesis. Chem. Eng. Sci. 1990, 45, 773–783. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-gas Shift: I. Intrinsic Kinetics. AlChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Wolfram, S. Mathematica: A System for Doing Mathematics by Computer; Addison-Wesley: Reading, MA, USA, 1991. [Google Scholar]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2003, 6, 1711. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Patterson, B.D.; Mo, F.; Borgschulte, A.; Hillestad, M.; Joos, F.; Kristiansen, T.; Sunde, S.; van Bokhoven, J.A. Renewable CO2 recycling and synthetic fuel production in a marine environment. Proc. Natl. Acad. Sci. USA 2019, 116, 12212–12219. [Google Scholar] [CrossRef] [Green Version]
- Miguel, C.V.; Soria, M.A.; Mendes, A.; Madeira, L.M. Direct CO2 hydrogenation to methane or methanol from post-combustion exhaust streams—A thermodynamic study. J. Nat. Gas Sci. Eng. 2015, 22, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62. [Google Scholar] [CrossRef]
- Moioli, E.; Mutschler, R.; Züttel, A. Renewable energy storage via CO2 and H2 conversion to methane and methanol: Assessment for small scale applications. Renew. Sustain. Energy Rev. 2019, 107, 497–506. [Google Scholar]
- Atkins, P.W.; de Paula, J. Physikalische Chemie; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Schüth, F. Chemical Compounds for Energy Storage. Chem. Ing. Tech. 2011, 83, 1984–1993. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Rush, J.D. Reduction potential of the carbon dioxide/carbon dioxide radical anion: A comparison with other C1 radicals. J. Phys. Chem. 1987, 91, 4429–4430. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics; Taylor & Francis: Abdingdon, UK, 2020. [Google Scholar]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni-Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Skrzypek, J.; Lachowska, M.; Serafin, D. Methanol Synthesis from CO2 and H2: Dependence of equilibrium conversions and exit equilibrium concentrations of components on the main process variables. Chem. Eng. Sci. 1990, 45, 89–96. [Google Scholar] [CrossRef]
- Stangeland, K.; Li, H.; Yu, Z. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols. Ind. Eng. Chem. Res. 2018, 57, 4081–4094. [Google Scholar] [CrossRef]
- Schlereth, D.; Hinrichsen, O. A fixed-bed reactor modeling study on the methanation of CO2. Chem. Eng. Res. Des. 2014, 92, 702–712. [Google Scholar]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar]
- Hanefeld, U.; Lefferts, L. Catalysis: An Integrated Textbook for Students; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Nørskov, J.K.; Studt, F.; Abild-Pedersen, F.; Bligaard, T. Fundamental Concepts in Heterogeneous Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Toyir, J.; Miloua, R.; Elkadri, N.E.; Nawdali, M.; Toufik, H.; Miloua, F.; Saito, M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst. Phys. Procedia 2009, 2, 1075–1079. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, R.; Bansode, A.; Urakawa, A. High-pressure advantages in stoichiometric hydrogenation of carbon dioxide to methanol. J. Catal. 2016, 343, 127–132. [Google Scholar]
- Slotboom, Y.; Bos, M.J.; Pieper, J.; Vrieswijk, V.; Likozar, B.; Kersten, S.R.A.; Brilman, D.W.F. Critical assessment of steady-state kinetic models for the synthesis of methanol over an industrial Cu/ZnO/Al2O3 catalyst. Chem. Eng. J. 2020, 389, 124181. [Google Scholar]
- Cao, H.; Wang, W.; Cui, T.; Zhu, G.; Ren, X. Enhancing CO2 hydrogenation to methane by Ni-based catalyst with V species using 3D-mesoporous KIT-6 as support. Energies 2020, 13, 2235. [Google Scholar] [CrossRef]
- Van-Dal, E.S.; Bouallou, C. Design and simulation of a methonal production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar]
- Falconer, J.L. Comparing CSTR and PFR Mass Balances, LearnChemE Video Presentation, Univ. Colorado, Boulder, and Private Communication. 2019. Available online: www.youtube.com/watch?v=xrOdRKzlkcE (accessed on 15 November 2020).
- Terreni, J.; Trottmann, M.; Franken, T.; Heel, A.; Borgschulte, A. Sorption-Enhanced Methanol Synthesis. Energy Technol. 2019, 7, 1801093. [Google Scholar] [CrossRef]















| ΔH0 [kJ/mol] | S0 [J/mol K] | |
|---|---|---|
| CO | −110.52 | 197.67 |
| CO2 | −393.51 | 213.74 |
| H2 | 0 | 130.68 |
| H2O | −241.82 | 188.82 |
| CH3OH | −200.66 | 239.81 |
| CH4 | −74.81 | 186.26 |
| Variable | a | b |
|---|---|---|
| KCO | 2.16 | 0.468 |
| KCO2 | 7.05 | 0.617 |
| KH2O/KH21/2 | 6.37 | 0.840 |
| Parameter | Methanol Synthesis | Methane Synthesis |
|---|---|---|
| Catalyst | Cu/ZnO/Al2O3 | Ni/MgAl2O4 |
| Catalyst density ρcatalyst | 1000 kg/m3 | 1000 kg/m3 |
| Nr of parallel tubes ntubes | 10,000 | 10 |
| Tube diameter d | 2 cm | 2 cm |
| Tube area Atube = ntube × πd2/4 | 3.14 m2 | 0.00314 m2 |
| Tube length Ltube | 3 m | 1 m |
| Initial flow velocity vflow | 0.05 m/s | 5 m/s |
| Stoichiometric number SN | 2 | 3 |
| Temperature range T | 180–340 °C | 100–1000 °C |
| Pressures P | 20, 40, 60 bar | 1, 10, 20 bar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terreni, J.; Borgschulte, A.; Hillestad, M.; Patterson, B.D. Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction. ChemEngineering 2020, 4, 62. https://doi.org/10.3390/chemengineering4040062
Terreni J, Borgschulte A, Hillestad M, Patterson BD. Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction. ChemEngineering. 2020; 4(4):62. https://doi.org/10.3390/chemengineering4040062
Chicago/Turabian StyleTerreni, Jasmin, Andreas Borgschulte, Magne Hillestad, and Bruce D. Patterson. 2020. "Understanding Catalysis—A Simplified Simulation of Catalytic Reactors for CO2 Reduction" ChemEngineering 4, no. 4: 62. https://doi.org/10.3390/chemengineering4040062