Clean Syn-Fuels via Hydrogenation Processes: Acidity–Activity Relationship in O-Xylene Hydrotreating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalytic Materials
2.2. Catalyst Characterization
2.3. Catalytic Study
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Catalytic Study
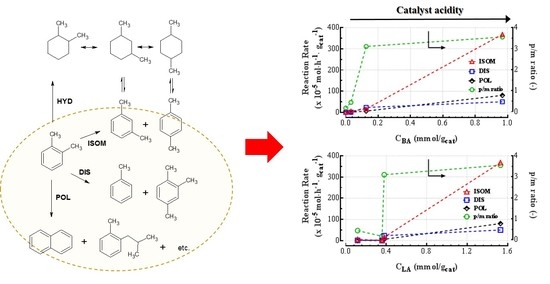
3.3. Acidity–Activity Relationships
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CEC DIRECTIVE 2009/30/EC of the European Parliament and of the Council. Off. J. Eur. Union 2009, 136–148. [CrossRef]
- Guo, R.; Cao, Z.; Fang, X. The development of catalysts and their stacking technology for diesel ultra-deep hydrosulfurization. Catal. Today 2018, 316, 21–25. [Google Scholar] [CrossRef]
- Pereyma, V.Y.; Klimov, O.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Yashnik, S.A.; Noskov, A.S. Effect of thermal treatment on morphology and catalytic performance of NiW/Al2O3 catalysts prepared using citric acid as chelating agent. Catal. Today 2018, 305, 162–170. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Mozhaev, A.V.; Maslakov, K.I.; Pimerzin, A.A.; Kogan, V.M. Genesis of HDT catalysts prepared with the use of Co2Mo10HPA and cobalt citrate: Study of their gas and liquid phase sulfidation. Appl. Catal. B Environ. 2014, 158–159, 161–174. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Salnikov, V.A.; Mozhaev, A.V.; Minaev, P.P.; Kogan, V.M.; Pimerzin, A.A. Relationship between active phase morphology and catalytic properties of the carbon-alumina-supported Co(Ni)Mo catalysts in HDS and HYD reactions. J. Catal. 2014, 309, 386–396. [Google Scholar] [CrossRef]
- Spadaro, L.; Palella, A.; Frusteri, F.; Arena, F. Valorization of crude bio-oil to sustainable energy vector for applications in cars powering and on-board reformers via catalytic hydrogenation. Int. J. Hydrogen Energy 2015, 40, 14507–14518. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Nishi, M.; Mochizuki, T.; Takagi, H.; Takatsuki, A.; Roschat, W.; Toba, M.; Yoshimura, Y. Co-processing of Jatropha-derived bio-oil with petroleum distillates over Mesoporous CoMo and NiMo Sulfide Catalysts. Catalysts 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Díaz de León, J.; Ramesh Kumar, C.; Antúnez-García, J.; Fuentes-Moyado, S. Recent Insights in Transition Metal Sulfide Hydrodesulfurization Catalysts for the Production of Ultra Low Sulfur Diesel: A Short Review. Catalysts 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhu, H.; Zhao, J.; Pan, L.; Dai, P.; Gu, X.; Li, L.; Liu, Y.; Zhao, X. Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization. Materials 2018, 11, 1067. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Garrido, I.; López-Benítez, A.; Berhault, G.; Guevara-Lara, A. Effect of support on the acidity of NiMo/Al2O3-MgO and NiMo/TiO2-Al2O3 catalysts and on the resulting competitive hydrodesulfurization/hydrodenitrogenation reactions. Fuel 2019, 236, 55–64. [Google Scholar] [CrossRef]
- Besenbacher, F.; Brorson, M.; Clausen, B.S.; Helveg, S.; Hinnemann, B.; Kibsgaard, J.; Lauritsen, J.V.; Moses, P.G.; Nørskov, J.K.; Topsøe, H. Recent STM, DFT and HAADF-STEM studies of sulfide-based hydrotreating catalysts: Insight into mechanistic, structural and particle size effects. Catal. Today 2008, 130, 86–96. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Bollinger, M.V.; Lægsgaard, E.; Jacobsen, K.W.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Besenbacher, F. Atomic-scale insight into structure and morphology changes of MoS2 nanoclusters in hydrotreating catalysts. J. Catal. 2004, 221, 510–522. [Google Scholar] [CrossRef]
- Breysse, M.; Geantet, C.; Afanasiev, P.; Blanchard, J.; Vrinat, M. Recent studies on the preparation, activation and design of active phases and supports of hydrotreating catalysts. Catal. Today 2008, 130, 3–13. [Google Scholar] [CrossRef]
- Topsøe, H. The role of Co-Mo-S type structures in hydrotreating catalysts. Appl. Catal. A Gen. 2007, 322, 3–8. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Nyberg, M.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; Besenbacher, F. Hydrodesulfurization reaction pathways on MoS2 nanoclusters revealed by scanning tunneling microscopy. J. Catal. 2004, 224, 94–106. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Guan, Z.; Li, Q.; He, C.; Yang, J. Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl. Catal. B Environ. 2017, 206, 300–307. [Google Scholar] [CrossRef]
- Nan, F.; Song, C.; Zhang, J.; Hui, R.; Chen, J.; Fairbridge, C.; Botton, G.A. STEM HAADF Tomography of Molybdenum Disulfide with Mesoporous Structure. ChemCatChem 2011, 3, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Ray, S.S.; Singh, I.D. Structural characterization of coke on spent hydroprocessing catalysts used for processing of vacuum gas oils. Appl. Catal. A Gen. 2004, 278, 83–91. [Google Scholar] [CrossRef]
- Furimsky, E.; Massoth, F.E. Deactivation of hydroprocessing catalysts. Catal. Today 1999, 52, 381–495. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Influence of catalyst acidity and feedstock quality on hydrotreating catalyst deactivation by coke deposition. Pet. Sci. Technol. 2001, 19, 697–710. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Effect of initial coking on hydrotreating catalyst functionalities and properties. Appl. Catal. A Gen. 1997, 159, 259–267. [Google Scholar] [CrossRef]
- Wiwel, P.; Zeuthen, P.; Jacobsen, A.C. Initial coking and deactivation of hydrotreating catalysts by real feeds. Stud. Surf. Sci. Catal. 1991, 68, 257–264. [Google Scholar] [CrossRef]
- Maity, S.K.; Blanco, E.; Ancheyta, J.; Alonso, F.; Fukuyama, H. Early stage deactivation of heavy crude oil hydroprocessing catalysts. Fuel 2012, 100, 17–23. [Google Scholar] [CrossRef]
- Lebreton, R.; Brunet, S.; Pérot, G.; Harlé, V.; Kasztelan, S. Deactivation and characterization of hydrotreating NiMo/Al2O3 catalyst coked by anthracene. In Catalyst Deactivation; Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 195–201. [Google Scholar]
- Scaroni, A.W.; Jenkins, R.G.; Utrilla, J.R.; Walker, P.L. Lewis acidity and coking of hydrodesulfuration catalysts. Fuel Process. Technol. 1984, 9, 103–108. [Google Scholar] [CrossRef]
- Lewandowski, M.; Sarbak, Z. Hydrofining activity and acid-base properties of nickel-molybdenum catalysts incorporated on sodium and magnesium ions-modified alumina. Appl. Catal. A Gen. 1998, 168, 179–185. [Google Scholar] [CrossRef]
- Marques, J.; Guillaume, D.; Merdrignac, I.; Espinat, D.; Brunet, S. Effect of catalysts acidity on residues hydrotreatment. Appl. Catal. B Environ. 2011, 101, 727–737. [Google Scholar] [CrossRef]
- Leyva, C.; Ancheyta, J.; Travert, A.; Maugé, F.; Mariey, L.; Ramírez, J.; Rana, M.S. Activity and surface properties of NiMo/SiO2-Al2O3 catalysts for hydroprocessing of heavy oils. Appl. Catal. A Gen. 2012, 425–426, 1–12. [Google Scholar] [CrossRef]
- Rana, M.S.; Huidobro, M.L.; Ancheyta, J.; Gómez, M.T. Effect of support composition on hydrogenolysis of thiophene and Maya crude. Catal. Today 2005, 107, 346–354. [Google Scholar] [CrossRef]
- Chen, W.; Maugé, F.; Van Gestel, J.; Nie, H.; Li, D.; Long, X. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts. J. Catal. 2013, 304, 47–62. [Google Scholar] [CrossRef]
- Rana, M.S.; Ancheyta, J.; Maity, S.K.; Rayo, P. Hydrotreating of maya crude oil: I. Effect of support composition and its pore-diameter on asphaltene conversion. Pet. Sci. Technol. 2007, 25, 187–199. [Google Scholar] [CrossRef]
- Kaluža, L.; Gulková, D.; Vít, Z.; Zdražil, M. High-activity MgO-supported CoMo hydrodesulfurization catalysts prepared by non-aqueous impregnation. Appl. Catal. B Environ. 2015, 162, 430–436. [Google Scholar] [CrossRef]
- Kaluža, L. Activity of transition metal sulfides supported on Al2O3, TiO2 and ZrO2 in the parallel hydrodesulfurization of 1-benzothiophene and hydrogenation of 1-methyl-cyclohex-1-ene. React. Kinet. Mech. Catal. 2015, 114, 781–794. [Google Scholar] [CrossRef]
- Giraldo, S.A.; Centeno, A. Isomerization and cracking under HDS conditions using γ-alumina modified with boron as catalysts support. Catal. Today 2008, 133, 255–260. [Google Scholar] [CrossRef]
- Huo, Q.; Dou, T.; Zhao, Z.; Pan, H. Synthesis and application of a novel mesoporous zeolite L in the catalyst for the HDS of FCC gasoline. Appl. Catal. A Gen. 2010, 381, 101–108. [Google Scholar] [CrossRef]
- Pérez-Martínez, D.J.; Eloy, P.; Gaigneaux, E.M.; Giraldo, S.A.; Centeno, A. Study of the selectivity in FCC naphtha hydrotreating by modifying the acid-base balance of CoMo/γ-Al2O3 catalysts. Appl. Catal. A Gen. 2010, 390, 59–70. [Google Scholar] [CrossRef]
- Guisnet, M.; Thomazeau, C.; Lemberton, J.L.; Mignard, S. Model reaction for the inSitu Characterization of the hydrogenative and acid properties of Industrial Hydrocracking Catalysts. J. Catal. 1995, 151, 102–110. [Google Scholar] [CrossRef]
- Watanabe, K.; Oshio, N.; Kawakami, T.; Kimura, T. Isomerization reactions with sulfur-containing pentane over Metal/SO42−/ZrO2 catalysts. Appl. Catal. A Gen. 2004, 272, 281–287. [Google Scholar] [CrossRef]
- Shi, G.; Fang, D.; Shen, J. Hydroisomerization of model FCC naphtha over sulfided Co(Ni)-Mo(W)/MCM-41 catalysts. Microporous Mesoporous Mater. 2009, 120, 339–345. [Google Scholar] [CrossRef]
- Karim, A.H.; Triwahyono, S.; Jalil, A.A.; Hattori, H. WO3 monolayer loaded on ZrO2: Property–activity relationship in n-butane isomerization evidenced by hydrogen adsorption and IR studies. Appl. Catal. A Gen. 2012, 433, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.D.; Barton, D.G.; Baertsch, C.D.; Iglesia, E. Reaction and Deactivation Pathways in Xylene Isomerization on Zirconia Modified by Tungsten Oxide. J. Catal. 2000, 194, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Zuo, D.; Li, D.; Nie, H.; Shi, Y.; Lacroix, M.; Vrinat, M. Acid-base properties of NiW/Al2O3 sulfided catalysts: Relationship with hydrogenation, isomerization and hydrodesulfurization reactions. J. Mol. Catal. A Chem. 2004, 211, 179–189. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Morin, S. Mechanisms of xylene isomerization over acidic solid catalysts. Microporous Mesoporous Mater. 2000, 35, 47–59. [Google Scholar] [CrossRef]
- Spadaro, L.; Arena, F.; Di Chio, R.; Palella, A. Definitive Assessment of the Level of Risk of Exhausted Catalysts: Characterization of Ni and V Contaminates at the Limit of Detection. Top. Catal. 2019, 62, 266–272. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Chai, Y.; Zhao, J.; Li, Y.; Liu, D.; Liu, Y.; Liu, C. Effect of sulfiding conditions on the hydrodesulfurization performance of the ex-situ presulfided CoMoS/γ-Al2O3 catalysts. Fuel 2018, 234, 1144–1153. [Google Scholar] [CrossRef]
- Penkova, A.; Bobadilla, L.F.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Pyridine adsorption on NiSn/MgO–Al2O3: An FTIR spectroscopic study of surface acidity. Appl. Surf. Sci. 2014, 317, 241–251. [Google Scholar] [CrossRef]
- Mochizuki, T.; Itou, H.; Toba, M.; Miki, Y.; Yoshimura, Y. Effects of Acidic Properties on the Catalytic Performance of CoMo Sulfide Catalysts in Selective Hydrodesulfurization of Gasoline Fractions. Energy Fuels 2008, 22, 1456–1462. [Google Scholar] [CrossRef]
- Franklin, J.L.; Nicholson, D.E. A Kinetic Study of the Decomposition of Hydrocarbons by Silica–Alumina Catalysts. J. Phys. Chem. 1956, 60, 59–62. [Google Scholar] [CrossRef]
- Guichard, B. Life Cycle of an HDT Catalyst. In Catalysis by Transition Metal Sulfides; Thoulhoat, H., Raybaud, P., Eds.; Editions Technip: Paris, France, 2013; pp. 301–357. ISBN 9782710809913. [Google Scholar]





| Catalyst | Composition (wt%) | S.A.BET (m2/g) | A.P.D. (nm) | P.V. (cm3/g) | CLA * (mmol/gcat) | CBA ** (mmol/gcat) | |||
|---|---|---|---|---|---|---|---|---|---|
| CoS or NiS | MoS2 | Al2O3 | SiO2 | ||||||
| CoMoSx | 7.29 | 27.5 | 65.2 | - | 215 | 8.0 | 0.33 | 0.380 | 0.126 |
| NiMoSx | 14.0 | 10.0 | 76.0 | - | 102 | 9.1 | 0.28 | 0.117 | 0.032 |
| LaY | - | - | 56.5 | 38.6 | 450 | 2.5 | 0.30 | 1.531 | 0.968 |
| -Al2O3 | - | - | 100 | - | 242 | 8.8 | 0.50 | 0.330 | 0.029 |
| Catalyst | Χo-xylene (%) | Reaction Rates (×10−5 mol·h−1∙gcat−1) | p-/m- | |||
|---|---|---|---|---|---|---|
| ISOM | HYD | DIS | POL | |||
| LaY | 47.4 | 368 | 0.00 | 50.3 | 80.7 | 3.55 |
| CoMoSx | 5.33 | 16.3 | 15.8 | 23.4 | 6.50 | 3.10 |
| NiMoSx | 1.81 | 6.26 | 11.4 | 1.22 | 1.67 | 0.44 |
| γ-Al2O3 | 0.37 | 1.73 | 0.00 | 0.19 | 1.88 | 0.17 |
| Catalyst | Χo-xylene (%) | Reaction Rates (×10−5 mol·h−1∙gcat−1) | p-/m- | ||
|---|---|---|---|---|---|
| ISOM | HYD | DIS | |||
| CoMoSx | 0.79 | 2.32 | 0.00 | 0.00 | 0.23 |
| NiMoSx | 0.60 | 2.29 | 0.00 | 0.00 | 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palella, A.; Barbera, K.; Arena, F.; Spadaro, L. Clean Syn-Fuels via Hydrogenation Processes: Acidity–Activity Relationship in O-Xylene Hydrotreating. ChemEngineering 2020, 4, 4. https://doi.org/10.3390/chemengineering4010004
Palella A, Barbera K, Arena F, Spadaro L. Clean Syn-Fuels via Hydrogenation Processes: Acidity–Activity Relationship in O-Xylene Hydrotreating. ChemEngineering. 2020; 4(1):4. https://doi.org/10.3390/chemengineering4010004
Chicago/Turabian StylePalella, Alessandra, Katia Barbera, Francesco Arena, and Lorenzo Spadaro. 2020. "Clean Syn-Fuels via Hydrogenation Processes: Acidity–Activity Relationship in O-Xylene Hydrotreating" ChemEngineering 4, no. 1: 4. https://doi.org/10.3390/chemengineering4010004