Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies
Abstract
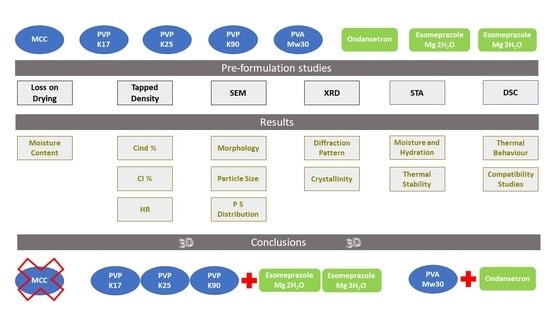
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Loss on Drying
2.2.2. Flowability—Tapped Density of Powders
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. X-ray Powder Diffraction (X-RPD)
2.2.5. Simultaneous Thermal Analysis (STA)
3. Results and Discussion
3.1. Moisture Content and Flowability
3.2. Morphology and Particle Size Distribution
3.3. Crystallinity
3.4. Stability and Thermal Profile
3.5. Compatibility between Raw Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lim, S.Y.; Pettit, R.S. Pharmacokinetic considerations in pediatric pharmacotherapy. Am. J. Health-Syst. Pharm. 2019, 76, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, D.K., Jr.; Smith, P.B.; Sun, M.J.; Murphy, M.D.; Avant, D.; Mathis, L.; Rodriguez, W.; Califf, R.M.; Li, J.S. Safety and transparency of pediatric drug trials. Arch. Pediatr. Adolesc. Med. 2009, 163, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, E.; Perez, R.; Hernandez, A.; Tejada, P.; Arteta, M.; Ramos, J.T. Factors and Mechanisms for Pharmacokinetic Differences between Pediatric Population and Adults. Pharmaceutics 2011, 3, 53–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germovsek, E.; Barker, C.I.; Sharland, M.; Standing, J.F. Scaling clearance in paediatric pharmacokinetics: All models are wrong, which are useful? Br. J. Clin. Pharm. 2017, 83, 777–790. [Google Scholar] [CrossRef] [Green Version]
- Schijvens, A.M.; de Wildt, S.N.; Schreuder, M.F. Pharmacokinetics in children with chronic kidney disease. Pediatr. Nephrol. 2020, 35, 1153–1172. [Google Scholar] [CrossRef] [Green Version]
- El-Rachidi, S.; LaRochelle, J.M.; Morgan, J.A. Pharmacists and Pediatric Medication Adherence: Bridging the Gap. Hosp. Pharm. 2017, 52, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Litalien, C.; Bérubé, S.; Tuleu, C.; Gilpin, A.; Landry, É.K.; Valentin, M.; Strickley, R.; Turner, M.A. From paediatric formulations development to access: Advances made and remaining challenges. Br. J. Clin. Pharmacol. 2022, 88, 4349–4383. [Google Scholar] [CrossRef]
- Polonini, H.C.; Silva, S.L.; Loures, S.; Almy, R.; Balland, A.; Brandão, M.A.F.; Ferreira, A.O. Compatibility of proton pump inhibitors in a preservative-free suspending vehicle. Eur. J. Hosp. Pharm. 2018, 25, 150–156. [Google Scholar] [CrossRef]
- Dean, L. Esomeprazole Therapy and CYP2C19 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kane, M.S., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Litalien, C.; Théorêt, Y.; Faure, C. Pharmacokinetics of proton pump inhibitors in children. Clin. Pharm. 2005, 44, 441–466. [Google Scholar] [CrossRef]
- Kader, R.; Liminga, G.; Ljungman, G.; Paulsson, M. Manipulations of Oral Medications in Paediatric Neurology and Oncology Care at a Swedish University Hospital: Health Professionals’ Attitudes and Sources of Information. Pharmaceutics 2021, 13, 1676. [Google Scholar] [CrossRef]
- Griddine, A.; Bush, J.S. Ondansetron; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sheshala, R.; Khan, N.; Chitneni, M.; Darwis, Y. Formulation and in vivo evaluation of ondansetron orally disintegrating tablets using different superdisintegrants. Arch. Pharmacal Res. 2011, 34, 1945–1956. [Google Scholar] [CrossRef]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbit, B.; Alvarez, J.C.; Dasque, E.; Abe, E.; Démolis, J.L.; Funck-Brentano, C. Droperidol and ondansetron-induced QT interval prolongation: A clinical drug interaction study. Anesthesiology 2008, 109, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardile, S.; Romano, C. Clinical utility of esomeprazole for treatment of gastroesophageal reflux disease in pediatric and adolescent patients. Adolesc. Health Med. 2012, 3, 27–31. [Google Scholar]
- Silva, M.R.M.d.; Dysars, L.P.; dos Santos, E.P.; Ricci Júnior, E. Preparation of extemporaneous oral liquid in the hospital pharmacy. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Martins, E.S.; Pereira, F.d.P.; Silva, L.T.M.d.; Beraldo, V.G.; Atique, T.S.C. Unitarização De Doses Em Farmácia Hospitalar. Infarma 2012, 24, 9–16. [Google Scholar]
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef]
- Carayon, P.; Wood, K.E. Patient safety—The role of human factors and systems engineering. Stud. Health Technol. Inform. 2010, 153, 23–46. [Google Scholar]
- Hsiao, W.K.; Lorber, B.; Reitsamer, H.; Khinast, J. 3D printing of oral drugs: A new reality or hype? Expert Opin. Drug Deliv. 2018, 15, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shiwpursad, J.; Xue, J. Comparison of Different Types of 3D Printing Technologies. Int. J. Sci. Res. 2018, 8, 1–9. [Google Scholar]
- Varghese, R.; Sood, P.; Salvi, S.; Karsiya, J.; Kumar, D. 3D printing in the pharmaceutical sector: Advances and evidences. Sens. Int. 2022, 3, 100177. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Jin, H.-E. Three-Dimensional Printing for Oral Pharmaceutical Dosage Forms. J. Pharm. Investig. 2022, 52, 293–317. [Google Scholar] [CrossRef]
- Nuñez, P.J.; Rivas, A.; García-Plaza, E.; Beamud, E.; Sanz-Lobera, A. Dimensional and Surface Texture Characterization in Fused Deposition Modelling (FDM) with ABS plus. Procedia Eng. 2015, 132, 856–863. [Google Scholar] [CrossRef]
- Chaurasia, G. A review on pharmaceutical preformulation studies in formulation and development of new drug molecules. Int. J. Pharm. Sci. Res. 2016, 7, 2313–2320. [Google Scholar]
- Deruyver, L.; Rigaut, C.; Lambert, P.; Haut, B.; Goole, J. The importance of pre-formulation studies and of 3D-printed nasal casts in the success of a pharmaceutical product intended for nose-to-brain delivery. Adv. Drug Deliv. Rev. 2021, 175, 113826. [Google Scholar] [CrossRef]
- Nogueira Prista, L.; Correia Alves, A.; Morgado, R.; Sousa Lobo, J. (Eds.) Tecnologia Farmacêutica, 6th ed.; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2003; Volume 1. [Google Scholar]
- Armstrong, B.; Brockbank, K.; Clayton, J. Understand the Effects of Moisture on Powder Behavior. Chem. Eng. Prog. 2014, 110, 25–30. [Google Scholar]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef]
- Sousa e Silva, J.P.; Splendor, D.; Goncalves, I.M.; Costa, P.; Sousa Lobo, J.M. Note on the measurement of bulk density and tapped density of powders according to the European Pharmacopeia. AAPS PharmSciTech 2013, 14, 1098–1100. [Google Scholar] [CrossRef] [Green Version]
- Hausner, H. Friction conditions in a mass of metal powder. Int. J. Powder Met. 1967, 3, 7–13. [Google Scholar]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical Properties of Poly-Vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Skieneh, J.; Khalili Najafabadi, B.; Horne, S.; Rohani, S. Crystallization of Esomeprazole Magnesium Water/Butanol Solvate. Molecules 2016, 21, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, R.; Uekusa, H. Elucidation of the Crystal Structures and Dehydration Behaviors of Ondansetron Salts. Crystals 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ganure, A.L.; Subudhi, B.B.; Shukla, S. Preparation and characterization of pH-sensitive methyl methacrylate-g-starch/hydroxypropylated starch hydrogels: In vitro and in vivo study on release of esomeprazole magnesium. Drug Deliv. Transl. Res. 2015, 5, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Panchamukhi, T. RSM-Based Design and Optimization of Transdermal Film of Ondansetron HCl. J. Pharm. Innov. 2020, 15, 94–109. [Google Scholar] [CrossRef]
- Azubuike, C.P.; Okhamafe, A.O. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int. J. Recycl. Org. Waste Agric. 2012, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Marian, E.; Tiţa, D. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J. Pharm. Biomed. Anal. 2011, 56, 221–227. [Google Scholar] [CrossRef]





| Moisture Content (%) | TGA 1st Mass Change (%) | CInd (%) | CI (%) | HR | |
|---|---|---|---|---|---|
| MCC | 8.00 ± 0.16 | <2.00 | 22.0 ± 1.3 | 21.8 ± 1.3 | 1.18 ± 0.03 |
| PVP K17 | 14.20 ± 0.28 | 12.36 | 16.3 ± 1.0 | 16.5 ± 1.2 | 1.15 ± 0.03 |
| PVP K25 | 15.70 ± 0.31 | 16.78 | 21.0 ± 1.2 | 21.0 ± 1.3 | 1.17 ± 0.02 |
| PVP K90 | 13.83 ± 0.22 | 12.25 | 17.0 ± 1.0 | 17.1 ± 1.2 | 1.09 ± 0.02 |
| PVA Mw30 | 5.30 ± 0.12 | 4.87 | 17.7 ± 1.1 | 17.2 ± 1.2 | 1.11 ± 0.03 |
| Esomeprazole Mg (2H2O) | 2.58 ± 0.10 | 5.19 | 23.4 ± 1.6 | 24.3 ± 1.3 | 1.26 ± 0.04 |
| Esomeprazole Mg (3H2O) | 2.97 ± 0.09 | 7.73 | 20.0 ± 1.5 | 20.1 ± 1.3 | 1.21 ± 0.03 |
| Ondansetron | 10.29 ± 0.22 | 9.59 | 17.0 ± 1.4 | 17.0 ± 1.2 | 1.11 ± 0.04 |
| Property | Circle Equivalent Diameter (µm) | |||
|---|---|---|---|---|
| Average | D10 | D50 | D90 | |
| MCC | 41.2 | 17.8 | 38.6 | 66.8 |
| PVP K17 | 66.0 | 26.6 | 64.2 | 104.0 |
| PVP K25 | 71.3 | 23.6 | 68.4 | 116.0 |
| PVP K90 | 143.0 | 63.7 | 142.0 | 224.0 |
| PVA Mw30 | 139.0 | 53.6 | 139.0 | 217.0 |
| Esomeprazole Mg (2H2O) | 6.7 | 2.7 | 6.1 | 12.0 |
| Esomeprazole Mg (3H2O) | 19.8 | 6.4 | 17.1 | 36.9 |
| Ondansetron | 114.0 | 41.0 | 101.0 | 208.0 |
| Melting Point (T °C) 1 | Decomposition (T °C) | |
|---|---|---|
| MCC | 338 | 300 |
| PVP K17 | 72 | 400 |
| PVP K25 | 82 | 400 |
| PVP K90 | 73 | 400 |
| PVA Mw30 | 180 | 280 |
| Esomeprazole Mg (2H2O) | 164 | 200 |
| Esomeprazole Mg (3H2O) | 149 | 180 |
| Ondansetron | 181 | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Lopes, C.M.; Gonçalves, H.; Pinto, J.F.; Catita, J. Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies. Appl. Sci. 2022, 12, 10585. https://doi.org/10.3390/app122010585
Ferreira M, Lopes CM, Gonçalves H, Pinto JF, Catita J. Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies. Applied Sciences. 2022; 12(20):10585. https://doi.org/10.3390/app122010585
Chicago/Turabian StyleFerreira, Mariana, Carla M. Lopes, Hugo Gonçalves, João F. Pinto, and José Catita. 2022. "Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies" Applied Sciences 12, no. 20: 10585. https://doi.org/10.3390/app122010585