Hepatoprotective Effect of the Ethanol Extract of Illicium henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. High-Performance Liquid Chromatography Coupled with Quadrupole Time of Flight Mass Spectrometry (HPLC–QTOF–MS) Analysis of EEIH
2.3. In Vitro Antioxidant Activity of EEIH
2.4. Experimental Animals and Treatment
2.5. Biochemical Examinations of Serum ALT and AST
2.6. Liver Histopathology Observation
2.7. Measurement of Cytokines
2.8. Measurement of MPO and SOD Activities and MDA, NO and Reduced GSH Levels in Liver Tissue
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. The Annotation and Structural Characterization of the Compounds in EEIH
3.2. In Vitro Antioxidant Activities of EEIH
3.3. Effect of EEIH on Liver Index, Serum ALT and AST Levels in LPS-Induced ALI Mice
3.4. Effects of EEIH on Liver Histopathology and MPO Activity in LPS-Induced ALI Mice
3.5. Effects of EEIH on the mRNA and Protein Expression Levels of Proinflammatory Factors in Liver Tissue of LPS-Induced ALI Mice
3.6. Effects of EEIH on the mRNA and Protein Expression Levels of TLR4 and NF-κB in Liver Tissue of LPS-Treated Mice
3.7. Effects of EEIH on Oxidative and Nitrosative Stress and the mRNA Expression Levels of Nrf2 in Liver Tissue of LPS-Treated Mice
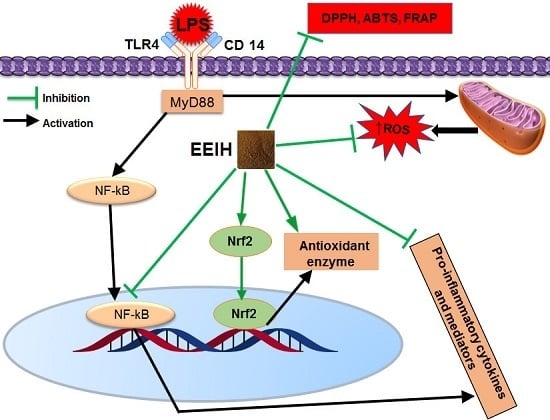
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Srivastava, B.; Gimson, A. Hepatic changes in systemic infection. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Bhondave, P.D.; Devarshi, P.P.; Mahadik, K.R.; Harsulkar, A.M. “Ashvagandharishta” prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. J. Ethnopharmacol. 2014, 151, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Gao, D.; Li, X.F.; Li, C.Y.; Li, R.S.; Zhao, Y.L.; Li, N.; Jia, G.L.; Pang, J.Y.; Cui, H.R.; et al. Inflammatory stress potentiates emodin-induced liver injury in rats. Front. Pharmacol. 2015, 6, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Liu, P.; Chen, Z.L.; Zhang, S.J.; Wang, Y.Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin attenuates lipopolysaccharide-induced acute liver injury via inhibiting the TLR4 signaling pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Kobashi, H.; Toshimori, J.; Yamamoto, K. Sepsis-associated liver injury: Incidence, classification and the clinical significance. Hepatol. Res. 2013, 43, 255–266. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Mendizabal, M.; Silva, M.O. Liver transplantation in acute liver failure: A challenging scenario. World. J. Gastroenterol. 2016, 22, 1523–1531. [Google Scholar] [CrossRef]
- Sha, J.; Zhang, H.; Zhao, Y.; Feng, X.; Hu, X.; Wang, C.; Song, M.; Fan, H. Dexmedetomidine attenuates lipopolysaccharide-induced liver oxidative stress and cell apoptosis in rats by increasing GSK-3beta/MKP-1/Nrf2 pathway activity via the α2 adrenergic receptor. Toxicol. Appl. Pharmacol. 2019, 364, 144–152. [Google Scholar] [CrossRef]
- Singh, A.; Koduru, B.; Carlisle, C.; Akhter, H.; Liu, R.M.; Schroder, K.; Brandes, R.P.; Ojcius, D.M. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4. Sci. Rep. 2017, 7, 14346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef] [PubMed]
- Latha, L.; Chaudhary, S.; Ray, S.R. Hydroalcoholic extract of Stevia rebaudiana Bert. leaves and stevioside ameliorates lipopolysaccharide induced acute liver injury in rats. Biomed. Pharmacother. 2017, 95, 1040–1050. [Google Scholar]
- Yang, Y.Q.; Yan, X.T.; Wang, K.; Tian, R.M.; Lu, Z.Y.; Wu, L.L.; Xu, H.T.; Wu, Y.S.; Liu, X.S.; Mao, W.; et al. Triptriolide alleviates lipopolysaccharide-induced liver injury by Nrf2 and NF-κB signaling pathways. Front. Pharmacol. 2018, 9, 999. [Google Scholar] [CrossRef]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The role of Nrf2 in liver disease: Novel molecular mechanisms and therapeutic approaches. Front. Pharmacol. 2018, 9, 1428. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective effects of Chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Gao, A.; Gong, J. An overview of pharmaceutical research on Illicium henryi Diels. J. Anhui Agric. Sci. 2011, 39, 8376–8377. [Google Scholar]
- Islam, M.S.; Miao, L.; Yu, H.; Han, Z.; Sun, H. Ethanol extract of Illicium henryi attenuates LPS-induced acute kidney injury in mice via regulating inflammation and oxidative stress. Nutrients 2019, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Jiang, Z.Y.; Zhang, Q.; Shi, Y.; Ma, Y.B.; Xie, M.J.; Zhang, X.M.; Chen, J.J. Henrylactones A–E and anti-HBV constituents from Illicium henryi. Planta Med. 2010, 76, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.J.; Ma, L.; Hu, L.H. Neolignans and flavonoids from the root bark of Illicium henryi. Fitoterapia 2010, 81, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Song, T.F.; Zhang, W.D.; Xia, X.H.; Shen, Y.H.; Liu, C.M.; Lin, S.; Jin, H.Z.; Li, H.L. Two new acorane sesquiterpenes from Illicium henryi. Arch. Pharm. Res. 2009, 32, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Jiang, Z.Y.; Geng, C.A.; Zhang, Q.; Shi, Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Two new lignans and anti-HBV constituents from Illicium henryi. Chem. Biodivers. 2011, 8, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.Y.; Zhang, G.J.; Wang, X.J.; Zhang, Y.; Yu, S.S.; Ma, S.G.; Liu, Y.B.; Qu, J.; Li, Y.; Xu, S.; et al. Prenylated C6-C3 compounds from the roots of Illicium henryi. Phytochemistry 2013, 86, 176–183. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Ni, T.T.; Liu, Z.Y.; Ye, Y.P.; Sun, H.X. Rapid annotation and structural characterization of saponins in the active fraction of Albizia julibrissin by HPLC coupled with quadrupole time-of-flight mass spectrometry based on accurate mass database. J. Sep. Sci. 2019, 42, 2922–2941. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry fruit extract affords protection against ethyl carbamate-induced cytotoxicity and oxidative stress. Oxid. Med. Cell Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, H.; Song, X.; Lu, M.; Zhao, L.; Xia, S.; You, G.; Zhao, J.; Zhang, Y.; Dong, A.; et al. Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials 2017, 144, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Song, T.F. Studies on the Chemical Constituents of Twigs and Leaves of Illicium henryi. Master’s Thesis, Hunan University of Traditional Chinese Medicine, Changsha, China, 2009. [Google Scholar]
- Gui, X.; Wang, G.; Zhang, N.; Huang, B. New phenylpropanoid and other compounds from Illicium lanceolatum with inhibitory activities against LPS-induced NO production in RAW 264.7 macrophages. Fitoterapia 2014, 95, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.D.; Zhu, H.Y.; Chen, Y.H.; Chen, W.S.; Xue, D.; Sun, L.N. Prenylated phenylpropanoid compounds from the stem bark of Illicium burmanicum. Fitoterapia 2015, 107, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gui, X. Study on the Chemical Contents and Anti-Gout Arthritis Activities of Illicium lanceolatum A.C. Smith Root Barks. Master’s Thesis, Fujian University of Traditional Chinese Medicine, Fuzhou, China, 2014. [Google Scholar]
- Ma, S.G.; Gao, Y.; Wang, H.Q.; Li, L.; Liu, Y.B.; Qu, J.; Li, Y.; Xu, S.; Lv, H.N.; Li, Y.H.; et al. Antiviral mono- and bis-prenylated C6-C3 derivatives from the roots of Illicium oligandrum. Tetrahedron 2016, 72, 3003–3013. [Google Scholar] [CrossRef]
- Ye, F.M.; Xie, Y.G.; Ren, J.; Ye, J.; Guo, Y.G.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Six new dihydrobenzofuran lignans from the branches and leaves of Illicium wardii and their cytotoxic activities. Phytochem. Lett. 2016, 17, 263–269. [Google Scholar] [CrossRef]
- Wang, G.W. Chemical Constituents and Anti-Inflammatory Activities of the Stems and Leaves of Illicium lanceolatum A.C. Smith. Master’s Thesis, Second Military Medical University, Shanghai, China, 2009. [Google Scholar]
- Pan, Z.H.; Ning, D.S.; Huang, S.S.; Cheng, L.; Xia, M.W.; Peng, L.Y.; Li, D.P. Lignan glucosides from the stem barks of Illicium difengpi. Molecules 2016, 21, 607. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Nishikawa, Y.; Harada, K.; Oda, M.; Huang, J.M.; Domon, H.; Terao, Y.; Fukuyama, Y. Tetranorsesquiterpenoids and santalane-type sesquiterpenoids from Illicium lanceolatum and their antimicrobial activity against the oral pathogen Porphyromonas gingivalis. J. Nat. Prod. 2015, 78, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, Y.; Kou, X.; Jing, Y.; Sun, K.; Sheng, D.; Yu, G.; Yu, D.; Zhao, Q.; Zhao, X.; et al. The protective or damaging effect of tumor necrosis factor-α in acute liver injury is concentration-dependent. Cell Biosci. 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.B.; Sung, N.Y.; Park, J.N.; Yang, M.S.; Park, S.H.; Byun, E.H. Gamma-irradiated resveratrol negatively regulates LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2015, 25, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.B.; Ouyang, K.H.; Wu, G.Q.; Chen, H.; Xiong, L.; Liu, X.; Wang, N.; Wang, W.J. Hepatoprotective effect of flavonoid-enriched fraction from Cyclocarya paliurus leaves on LPS/D-GalN-induced acute liver failure. J. Funct. Foods 2018, 48, 337–350. [Google Scholar] [CrossRef]
- Ye, H.Y.; Wu, W.S.; Liu, Z.W.; Xie, C.F.; Tang, M.H.; Li, S.C.; Yang, J.H.; Tang, H.; Chen, K.; Long, C.F.; et al. Bioactivity-guided isolation of anti-inflammation flavonoids from the stems of Millettia dielsiana Harms. Fitoterapia 2014, 95, 154–159. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Zhai, W.; Zheng, J.; Yao, X.; Peng, B.; Liu, M.; Huang, J.; Wang, G.; Xu, Y. Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement. Altern. Med. 2013, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gulcin, I.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzyme. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Liang, L.; Gao, C.; Luo, M.; Wang, W.; Zhao, C.; Zu, Y.; Efferth, T.; Fu, Y. Dihydroquercetin (DHQ) induced HO-1 and NQO1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J. Agric. Food Chem. 2013, 61, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Zhu, P.; Fujino, M.; Takahara, T.; Toyama, S.; Tomita, A.; Zhao, L.; Yang, Z.; Hei, M.; et al. Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int. Immunopharmacol. 2015, 28, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Tselkin, Y.O.; Babenkova, I.V.; Kolhir, V.K.; Baginskaya, A.I.; Tjukavkina, N.A.; Kolesnik, Y.A.; Selivanova, I.A.; Eichholz, A.A. Dihydroquercetin as a means of antioxidative defence in rats with tetrachloromethane hepatitis. Phytother. Res. 2000, 14, 160–162. [Google Scholar] [CrossRef]
- Crown, O.O.; Ogundele, O.O.; Akinmoladun, A.C.; Famusiwa, C.D.; Josiah, S.S.; Olaleye, M.T.; Akindahunsi, A.A. Effects of catechin, quercetin and taxifolin on redox parameters and metabolites linked with renal health in rotenone-toxified rats. Niger. J. Physiol. Sci. 2019, 34, 1–10. [Google Scholar] [PubMed]
- Kwon, S.H.; Hong, S.I.; Ma, S.X.; Lee, S.Y.; Jang, C.G. 3′,4′,7-Trihydroxyflavone prevents apoptotic cell death in neuronal cells from hydrogen peroxide-induced oxidative stress. Food Chem. Toxicol. 2015, 80, 41. [Google Scholar] [CrossRef]
- Wei, S.D.; Li, J.Z.; Liu, Z.J.; Chen, Q.; Chen, Y.; Chen, M.; Gong, J.P. Dexamethasone attenuates lipopolysaccharide-induced liver injury by downregulating glucocorticoid-induced tumor necrosis factor receptor ligand in Kupffer cells. Hepatol. Res. 2011, 41, 989–999. [Google Scholar] [CrossRef]
- Hassan, H.M.; Guo, H.; Yousef, B.A.; Ping-Ping, D.; Zhang, L.; Jiang, Z. Dexamethasone pretreatment alleviates isoniazid/lipopolysaccharide hepatotoxicity: Inhibition of inflammatory and oxidative stress. Front. Pharmacol. 2017, 8, 133. [Google Scholar] [CrossRef]
- Gao, H.W.; Cui, Y.K.; Kang, N.X.; Liu, X.; Liu, Y.L.; Zou, Y.; Zhang, Z.Y.; Li, X.R.; Yang, S.L.; Li, J.; et al. Isoacteoside, a dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects through blocking toll-like receptor 4 dimerization. Br. J. Pharmacol. 2017, 174, 2880–2896. [Google Scholar] [CrossRef] [Green Version]
- Annane, D. Low-dose adrenocorticotropic hormone test is not ready for routine adrenal function testing in the intensive care unit. Crit. Care Med. 2005, 33, 2688–2689. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Han, X.; Tao, X.; Xu, L.; Xu, Y.; Fang, L.; Yin, L.; Qi, Y.; Li, H.; Peng, J. Protection by the total flavonoids from Rosa laevigata Michx fruit against lipopolysaccharide-induced liver injury in mice via modulation of FXR signaling. Foods 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji white tea polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Maksymchuk, O.; Shysh, A.; Rosohatska, I.; Chashchyn, M. Quercetin prevents type 1 diabetic liver damage through inhibition of CYP2E1. Pharmacol. Rep. 2017, 69, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Wang, J.; Zhou, W.; Sun, R.; Xia, M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2015, 102, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reber, L.L.; Gillis, C.M.; Starkl, P.; Jonsson, F.; Sibilano, R.; Marichal, T.; Gaudenzio, N.; Berard, M.; Rogalla, S.; Contag, C.H.; et al. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J. Exp. Med. 2017, 214, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Heinlein, S.; Agli, A.; Bang, R.; Schumann, J.; Tiegs, G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Yi, M.; Wang, R.; Huang, Y.; Chen, M. Protective effects of costunolide against D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Front. Pharmacol. 2018, 9, 1469. [Google Scholar] [CrossRef]
- Deng, X.; Wu, K.; Wan, J.; Li, L.; Jiang, R.; Jia, M.; Jing, Y.; Zhang, L. Aminotriazole attenuated carbon tetrachloride-induced oxidative liver injury in mice. Food Chem. Toxicol. 2012, 50, 3073–3078. [Google Scholar] [CrossRef]
- De Araújo Júnior, R.F.; Garcia, V.B.; Leitao, R.F.; Brito, G.A.; Miguel Ede, C.; Guedes, P.M.; de Araujo, A.A. Carvedilol improves inflammatory response, oxidative stress and fibrosis in the alcohol-induced liver injury in rats by regulating Kuppfer cells and hepatic stellate cells. PLoS ONE 2016, 11, e0148868. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Zhao, Y.; Cheng, H.; Zhao, Z.; Lei, G.; Niu, C.; Chao, W.; Liu, X.; Zhang, C.; Li, S. Hepatoprotective effects of Lactobacillus plantarum C88 on LPS/D-GalN–induced acute liver injury in mice. J. Funct. Foods 2018, 43, 146–153. [Google Scholar] [CrossRef]
- McGhan, L.J.; Jaroszewski, D.E. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury 2012, 43, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ari, Z.B.; Avlas, O.; Pappo, O.; Zilbermints, V.; Cheporko, Y.; Bachmetov, L.; Zemel, R.; Shainberg, A.; Sharon, E.; Grief, F.; et al. Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell. Physiol. Biochem. 2012, 29, 41–50. [Google Scholar] [PubMed]
- Sato, K.; Miyakawa, K.; Takeya, M.; Hattori, R.; Yui, Y.; Sunamoto, M.; Ichimori, Y.; Ushio, Y.; Takahashi, K. Immunohistochemical expression of inducible nitric oxide synthase (iNOS) in reversible endotoxic shock studied by a novel monoclonal antibody against rat iNOS. J. Leukoc. Biol. 1995, 57, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Boveris, A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Radic. Biol. Med. 2004, 37, 1472–1478. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 2002, 33, 1440–1450. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective effect of argan and olive oils against LPS-induced oxidative stress and inflammation in mice livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef]
- Zapelini, P.H.; Rezin, G.T.; Cardoso, M.R.; Ritter, C.; Klamt, F.; Moreira, J.C.; Streck, E.L.; Dal-Pizzol, F. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 2008, 8, 211–218. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.X.; Wan, R.Z.; Zhang, P.; Zhang, Q.Y.; Chen, Z.; Zhang, B.S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723–30733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galicia-Moreno, M.; Gutiérrez-Reyes, G. The role of oxidative stress in the development of alcoholic liver disease. Rev. Gastroenterol. Mex. 2014, 79, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- Kwak, M.K.; Wakabayashi, N.; Kensler, T.W. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat. Res. 2004, 555, 133–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Y.; Liu, C.; Li, Y.; Guo, W.; Wu, J.L.; Xu, D.; You, X.; Pan, Y.; Chen, Y. Identification of an adaptor protein that facilitates Nrf2-Keap1 complex formation and modulates antioxidant response. Free Radic. Biol. Med. 2016, 97, 38–49. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef]
- Qi, Z.; Yao, L.; Li, F.; Yong, T.; Jian, M. Polyphenols extracted from Coreopsis tinctoria buds exhibited a protective effect against acute liver damage. J. Funct. Foods 2018, 44, 201–208. [Google Scholar]











| Peaks | Rt (min) | Molecular Formula | QMI (m/z) | MS/MS Fragment Ions (Relative Abundance) | Annotated Compound | Ref. |
|---|---|---|---|---|---|---|
| 1 | 3.493 | C15H14O6 | [M + H]+ (291.0867) | 123.0431 (51.72), 139.0372 (100.00), 161.0607 (9.04), 207.0630 (10.39), 231.1076 (1.18), 258.1289 (0.76), 291.0867 (0.83) | (+)-Catechin (1) | [20] |
| 2 | 5.127 | C15H10O5 | [M + H]+ (271.0541) | 107.0439 (21.19), 137.0542 (100.00), 173.0532 (16.42), 197.0507 (13.28), 227.1040 (7.83), 271.0541 (9.38) | 3′,4′,7-Trihydroxyflavone (2) | [29] |
| 3 | 5.536 | C15H20O8 | [M + NH4]+ (346.1505) | 107.0464 (94.61), 147.0432 (100.00), 195.0804 (65.03), 222.0607 (85.62), 301.1067 (21.00), 346.1505 (9.21) | Anisatin (3), (2S)-Hydroxy- neomajucin (4), (2R)-2-hy- droxyneomajucin (5), majucin (6) | [19] |
| 4 | 6.288 | C15H12O7 | [M + H]+ (305.0667) | 111.0432 (4.00), 123.0432 (66.39), 153.0164 (100.00), 195.0260 (12.47), 231.0626 (87.29), 259.0575 (31.005), 287.0535 (31.05), 305.0667 (8.02) | Taxifolin (7) | [30] |
| 5 | 6.550 | C15H18O4 | [M + H]+ (263.1285) | 105.0665(29.06), 135.0775 (100.00), 159.1145 (47.49), 185.0860 (24.58), 203.1038(37.05), 227.1041 (18.19), 263.1285 (9.02) | Illihenryiones E (8), C (9), illifrognone D (10), burmanicumol D (11) | [23,31] |
| 6 | 7.155 | C8H8O3 | [M + H]+ (153.0609) | 107.0800 (100.00), 119.0787 (6.92), 135.1107 (26.23), 142.0674 (7.76), 153.0609 (15.98) | Vanillin (12) | [32] |
| 7 | 7.285 | C17H24O5 | [M + Na]+ (331.1550) | 119.0488 (13.31), 151.0727 (100.00), 189.0903 (32.84), 227.1042 (43.28), 255.0985 (31.55), 285.1108 (19.79), 331.1550 (2.16) | Illioliganones K (13) or E (14) | [31,33] |
| 8 | 7.547 | C21H32O10 | [M + NH4]+ (462.2341) | 133.0937 (100.00), 229.1092 (1.91), 287.1129 (1.83), 341.1190 (2.30), 409.1432 (0.48). 462.2341 (0.31) | Henrylactone D (15) | [19] |
| 9 | 8.773 | C27H34O11 | [M + NH4]+ (552.2448) | 137.0586 (88.95), 177.0886 (12.94), 237.1098 (20.37), 305.1507 (100.00), 355.1507 (100.00), 552.2448 (0.59) | Dihydrodehydrodiconiferyl alcohol 9-O-β-D-(3′-O-acetyl)-xylopyranoside (16) | [21] |
| 10 | 8.855 | C27H34O11 | [M + NH4]+ (552.2448) | 137.0522 (98.74), 177.0799 (16.41), 237.0996 (25.60), 305.1004 (33.79), 355.1346 (100.00), 415.0753 (1.05), 552.2448 (8.59) | Illiciumlignan F(17) | [34] |
| 11 | 9.296 | C15H18O3 | [M + H]+ (247.1336) | 146.0993 (100.00), 159.1164 (44.07), 173.1287 (58.84), 189.1254 (63.00), 213.1257 (21.07), 231.1357 (19.30), 247.1336 (22.20) | 1-Allyl-2-(3-methylbut-2-enyloxy)-4,5-methylenedioxybenzene (18) | [32] |
| 12 | 11.127 | C14H16O4 | [M + H]+ (249.1174) | 145.0940 (87.48), 163.1009 (57.58), 175.1011 (39.15), 191.1021 (33.87), 203.0928 (19.31), 215.1304 (11.00), 233.1397 (25.03), 249.1174 (5.89) | Trans-3-methoxy-4,5-methylene-dioxycinnamaldehyde (19) | [29] |
| 13 | 11.601 | C22H26O6 | [M + H]+ (387.1812) | 225.0888 (4.39), 253.0858 (11.04), 281.0829 (13.40), 323.1258 (51.40), 355.1517 (100.00), 387.1812 (2.42) | Tashironin (20) | [19] |
| 14 | 12.957 | C22H26O6 | [M + H]+ (387.1812) | 167.0681 (7.82), 211.0714 (6.48), 263.1026 (9.50), 323.1217 (52.67), 355.1476 (100.00), 387.1812(4.11) | Illioliganfuranol A (21) | [33] |
| 15 | 13.17 | C15H22O2 | [M + H]+ (235.1696) | 137.0890 (34.98), 159.1094 (87.25), 165.1200 (27.72), 184.1164 (6.44), 199.1412 (100.00), 217.1527 (12.93), 235.1578 (8.33), 235.1696 (3.09) | (−)-1-Hydroxy-1,3,5-bisabolatrien-10-one (22) | [33] |
| 16 | 13.301 | C22H24O6 | [M + H]+ (385.1654) | 165.0563 (5.89), 225.0901 (7.64), 263.1055 (12.81), 295.1332 (32.59), 316.0933 (97.08), 353.1380 (100.00), 385.1654 (73.07) | 4-Allyl-2,6-dimethoxyphenyl-3,4-dimethoxycinnamate (23) | [35] |
| 17 | 14.788 | C14H22O2 | [M + H]+ (223.1698) | 107.0796 (68.42), 121.0941 (100.00), 131.0833 (18.38), 147.1112 (83.63), 165.1207 (37.30), 178.0644 (8.49), 195.0740 (10.71), 210.0588 (5.76), 223.1698 (15.51) | 1β-Isopropyl-4-β-methyl-9β-hydroxy spiro [4,5] dec-6-en-8-one (24) | [29] |
| 18 | 14.919 | C15H24O2 | [M + H]+ (237.1852) | 133.0998 (26.34), 147.1158 (100.00), 161.1307 (49.35), 179.1424 (27.87), 201.1602 (9.95), 219.1723 (28.61), 237.1852 (1.94) | 10-Hydroxyacoronene (25) | [29] |
| 19 | 15.05 | C22H30O6 | [M + H]+ (391.2118) | 107.0435 (2.69), 145.0938 (5.85), 167.0635 (100.00), 205.1153 (59.58), 237.1415 (25.58), 391.2118 (1.55) | Tashironin A (26) | [30] |
| 20 | 17.404 | C26H36O9 | [M + Na]+ (515.2284) | 113.0543 (4.63), 184.0670 (2.57), 316.0877 (31.07), 385.1589 (100.00), 469.2170 (11.84), 469.2170 (11.84), 515.2284 (7.33) | (−)Secoisolariciresinol-O-α-L-rhamnopyranoside (27) | [36] |
| 21 | 19.889 | C20H24O6 | [M + Na]+ (383.1475) | 197.0903 (0.78), 225.0889 (2.61), 263.1051 (5.51), 314.0772 (98.58), 353.1369 (100.00), 368.1240 (1.84), 383.1475 (35.43) | (+)-Isolariciresinol (28) | [36] |
| 22 | 21.85 | C10H12O3 | [M + Na]+ (203.1733) | 105.0678 (100.00), 119.0832 (81.51), 133.0982 (43.59), 147.1136 (60.78), 161.1287 (20.74), 175.1407 (2.70), 203.1733 (12.13) | 2-(4-Hydroxyphenyl) ethyl acetate (29) | [29] |
| 23 | 23.550 | C15H22O | [M + H]+ (219.1746) | 109.1006 (100.00), 145.0999 (18.70), 173.1304 (5.94), 207.0295 (3.52),219.1746 (3.31) | α-Santal-11-en-10-one (30) | [37] |
| 24 | 24.727 | C7H6O2 | [M-H2 + H]+ (121.1019) | 102.0493 (2.04), 103.0555 (48.44), 105.0687 (100.00), 107.0820 (5.07), 115.0484 (3.19), 119.0838 (16.97), 121.1019 (14.47) | Hydroxybenzaldehyde (31) | [32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Yu, H.; Miao, L.; Liu, Z.; He, Y.; Sun, H. Hepatoprotective Effect of the Ethanol Extract of Illicium henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide. Antioxidants 2019, 8, 446. https://doi.org/10.3390/antiox8100446
Islam MS, Yu H, Miao L, Liu Z, He Y, Sun H. Hepatoprotective Effect of the Ethanol Extract of Illicium henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide. Antioxidants. 2019; 8(10):446. https://doi.org/10.3390/antiox8100446
Chicago/Turabian StyleIslam, Md Sodrul, Hui Yu, Lingyan Miao, Zhaoying Liu, Yanfei He, and Hongxiang Sun. 2019. "Hepatoprotective Effect of the Ethanol Extract of Illicium henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide" Antioxidants 8, no. 10: 446. https://doi.org/10.3390/antiox8100446