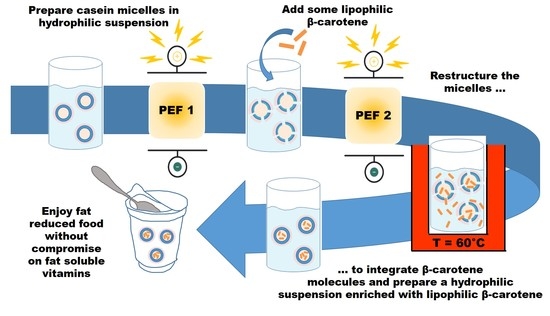
Affecting Casein Micelles by Pulsed Electrical Field (PEF) for Inclusion of Lipophilic Organic Compounds
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Casein Micelle Suspension
2.1.2. β-Carotene Solution
2.2. Methods
2.2.1. PEF Treatment
2.2.2. Analysis of β-Carotene Content
2.2.3. Determination of Particle Size Distribution (PSD) and Resulting Specific Surface Area (SSA)
2.2.4. Determination of Surface Charge and Charge Density: Surface Potential
2.2.5. Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussion
3.1. Disintegration of CM: Defining PEF Parameters
3.2. Loading Procedure and Restructuring of CM by Thermization
3.3. Restructuring of CM via PEF
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. WHO Fact Sheet No. 311. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/# (accessed on 8 April 2017).
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Keppler, J.; Schwarz, K. Milk proteins as nanotransporters. Eur. Diary Mag. 2012, 10–13. [Google Scholar]
- Bulca, S.; Leder, J.; Kulozik, U. Impact of UHT or high heat treatment on the rennet gel formation of skim milk with various whey protein content. Milchwissenschaft 2004, 59, 590–593. [Google Scholar]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Native casein micelles as nanocarriers for β-carotene: pH-and temperature-induced opening of the micellar structure. Int. J. Food Sci. Technol. 2017, 52, 1122–1130. [Google Scholar] [CrossRef]
- Knoop, A.-M.; Knoop, E.; Wiechen, A. Sub-structure of synthetic casein micelles. J. Dairy Res. 1979, 46, 347–350. [Google Scholar] [CrossRef]
- Semo, E.; Kesselman, E.; Danino, D.; Livney, Y. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll. 2007, 21, 936–942. [Google Scholar] [CrossRef]
- Haham, M.; Ish-Shalom, S.; Nodelman, M.; Duek, I.; Segal, E.; Kustanovich, M.; Livney, Y.D. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct. 2012, 3, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Aguirre, O.; Stuetz, W.; Grune, T.; Kessler, A.; Weiss, J.; Hinrichs, J. High pressure-assisted encapsulation of vitamin D2 in reassembled casein micelles. High Press. Res. 2011, 31, 265–274. [Google Scholar] [CrossRef]
- Schrader, K.; Morr, C.; Buchheim, W. High pressure effects on the colloidal calcium phosphate and the structural integrity of micellar casein in milk. Prog. Biotechnol. 1996, 13, 347–350. [Google Scholar]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. Dissociation of caseins in high pressure-treated bovine milk. Int. Dairy J. 2004, 14, 675–680. [Google Scholar] [CrossRef]
- Bourassa, P.; N’Soukpoé-Kossi, C.; Tajmir-Riahi, H. Binding of vitamin A with milk α- and β-caseins. Food Chem. 2013, 138, 444–453. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Nakagawa, K. Spray drying of casein aggregates loaded with β-carotene: Influences of acidic conditions and storage time on surface structure and encapsulation efficiencies. Dry Technol. 2013, 31, 1459–1465. [Google Scholar] [CrossRef]
- Nakagawa, K.; Jarunglumlert, T.; Adachi, S. Microencapsulation of β-carotene by self-aggregated caseinates. Jpn. J. Food Eng. 2014, 15, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Reimerdes, E.H.; Jimenezperez, S.; Ringqvist, B.M. Temperature-dependent changes in milk-products. 3. Thermization—A corrective step in processing of refrigerated milk. Milchwissenschaft 1977, 32, 207–210. [Google Scholar]
- Reimerdes, E.H. Changes in the proteins of raw milk during storage. Dev. Dairy Chem. 1982, 1, 271–288. [Google Scholar]
- Walstra, P. On the Stability of Casein Micelles. J. Dairy Sci. 1990, 73, 1965–1979. [Google Scholar] [CrossRef]
- Saulis, G.; Wouters, P. Probable mechanisms of microorganism inactivation by pulsed electric fields. Food Preserv. Pulsed Electr. Fields 2007, 2007, 138–155. [Google Scholar]
- Fauster, T.; Ostermeier, R.; Scheibelberger, R.; Jäger, H. Pulsed Electric Field (PEF) Application in the Potato Industry. Innov. Food Process. Technol. 2021, 2021, 253–270. [Google Scholar]
- Marsellés-Fontanet, A.R.; Elez-Martínez, P.; Martín-Belloso, O. Juice preservation by pulsed electric fields. Stewart Postharvest Rev. 2012, 8, 1–4. [Google Scholar]
- Toepfl, S.; Heinz, V. Mass Transport Improvement by PEF—Applications in the Area of Extraction and Distillation. Distill. Adv. Model. Appl. 2012, 2012, 211–232. [Google Scholar]
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chem. Eng. Process. Process. Intensif. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Calderón-Miranda, M.L.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Listeria innocua in liquid whole egg by pulsed electric fields and nisin. Int. J. Food Microbiol. 1999, 51, 7–17. [Google Scholar] [CrossRef]
- Picart, L.; Dumay, E.; Cheftel, J. Inactivation of Listeria innocua in dairy fluids by pulsed electric fields: Influence of electric parameters and food composition. Innov. Food Sci. Emerg. Technol. 2002, 3, 357–369. [Google Scholar] [CrossRef]
- Sampedro, F.; Rodrigo, M.; Martinez, A.; Barbosa-Cánovas, G.V.; Rodrigo, D. Quality and Safety Aspects of PEF Application in Milk and Milk Products. Crit. Rev. Food Sci. Nutr. 2005, 45, 25–47. [Google Scholar] [CrossRef]
- Sepulveda, D.; Góngora-Nieto, M.; Guerrero, J.; Barbosa-Cánovas, G. Production of extended-shelf life milk by processing pasteurized milk with pulsed electric fields. J. Food Eng. 2005, 67, 81–86. [Google Scholar] [CrossRef]
- Floury, J.; Grosset, N.; Leconte, N.; Pasco, M.; Madec, M.-N.; Jeantet, R. Continuous raw skim milk processing by pulsed electric field at non-lethal temperature: Effect on microbial inactivation and functional properties. Le Lait 2005, 86, 43–57. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Bendicho-Porta, S.; Martín-Belloso, O. Comparative Study on Shelf Life of Whole Milk Processed by High-Intensity Pulsed Electric Field or Heat Treatment. J. Dairy Sci. 2006, 89, 905–911. [Google Scholar] [CrossRef]
- Sobrino-López, Á.; Martín-Belloso, O. Enhancing Inactivation of Staphylococcus aureus in Skim Milk by Combining High-Intensity Pulsed Electric Fields and Nisin. J. Food Prot. 2006, 69, 345–353. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.Á.; Sepulveda, D.R.; Góngora-Nieto, M.M.; Swanson, B.; Barbosa-Cánovas, G.V. Milk thermization by pulsed electric fields (PEF) and electrically induced heat. J. Food Eng. 2010, 100, 56–60. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Fernández, S.; Esquivel, H.; Dunne, P.C.; Barbosa-Cánovas, G.V. Milk Processed by Pulsed Electric Fields: Evaluation of Microbial Quality, Physicochemical Characteristics, and Selected Nutrients at Different Storage Conditions. J. Food Sci. 2011, 76, S289–S299. [Google Scholar] [CrossRef] [PubMed]
- Bolshov, M.A.; Kuritsyn, Y.A. Laser Analytical Spectroscopy. Ullmann’s Encycl. Ind. Chem. 2001, 2012, 595–631. [Google Scholar]
- Dunn, J. Pulsed Light and Pulsed Electric Field for Foods and Eggs. Poult. Sci. 1996, 75, 1133–1136. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Pargmann, S.; Kurz, J.; Lorenzen, P.C. Comparative studies of loading lipophilic substances into casein micelles and investigating the influence of whey proteins and heat treatment on loading stability. Int. J. Dairy Technol. 2018, 71, 954–965. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik rüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 4, 377–445. [Google Scholar] [CrossRef]
- Kirchmei, O. Secondary Phase of Milk Coagulation by Rennin; 2. Changes in Electrochemical State of Micelle. Z. Lebensm. Unters. Forsch. 1972, 149, 211–215. [Google Scholar]
- Liu, Z. Effects of Pulsed Electric Field Processing and Ultrasound Processing on the Physicochemical and Functional Properties of Proteins in Milk. Ph.D. Thesis, RMIT University, Melbourne, Australia, August 2012. [Google Scholar]
- Puippe, J.C.; Ibl, N. Influence of charge and discharge of electric double layer in pulse plating. J. Appl. Electrochem. 1980, 10, 775–784. [Google Scholar] [CrossRef]
- Saiz-Abajo, M.J.; Gonzalez-Ferrero, C.; Moreno-Ruiz, A.; Romo-Hualde, A.; Gonzalez-Navarro, C.J. Thermal protection of β-carotene in re-assembled casein micelles during different processing technologies applied in food industry. Food Chem. 2013, 138, 1581–1587. [Google Scholar] [CrossRef]







| Control | PEF1-Treated | ||||
|---|---|---|---|---|---|
| ttherm | 15 | 0 | 15 | 30 | 45 |
| surface potential (mV) | −663 ± 28 | −638 ± 26 | −565 ± 29 | −473 ± 29 | −407 ± 31 |
| β-carotene content (µg/100g) | <5 | <5 | −22 ± 5 | −0.24 ± 6 | −51 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Middendorf, D.; Bindrich, U.; Siemer, C.; Töpfl, S.; Heinz, V. Affecting Casein Micelles by Pulsed Electrical Field (PEF) for Inclusion of Lipophilic Organic Compounds. Appl. Sci. 2021, 11, 4611. https://doi.org/10.3390/app11104611
Middendorf D, Bindrich U, Siemer C, Töpfl S, Heinz V. Affecting Casein Micelles by Pulsed Electrical Field (PEF) for Inclusion of Lipophilic Organic Compounds. Applied Sciences. 2021; 11(10):4611. https://doi.org/10.3390/app11104611
Chicago/Turabian StyleMiddendorf, Dana, Ute Bindrich, Claudia Siemer, Stefan Töpfl, and Volker Heinz. 2021. "Affecting Casein Micelles by Pulsed Electrical Field (PEF) for Inclusion of Lipophilic Organic Compounds" Applied Sciences 11, no. 10: 4611. https://doi.org/10.3390/app11104611