Degradation of Phenol by Immobilized Alcaligenes faecalis Strain JH1 in Fe3O4-Modified Biochar from Pharmaceutical Residues
Abstract
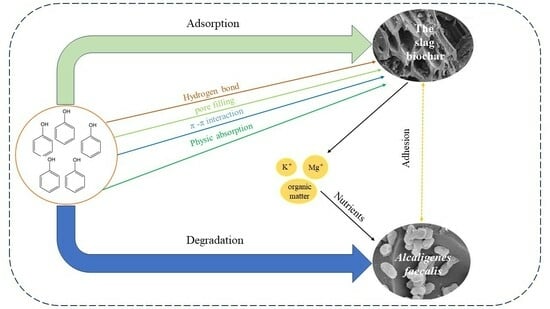
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Culture Medium
2.2. Fe3O4 Nanoparticle-Modified Biochar Preparation
2.3. Material Characterization
2.4. Immobilization of Strain JH1 to Biochar
2.5. Determination of the Microbial Biomass
2.6. Biosorption and Reusability Studies: Batch Experiments
2.7. Effect of Biochar Water-Extractable Organic Carbon on Strain JH1
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Scanning Electron Microscopy (SEM) Analysis
2.10. Analytical Methods of Phenol
3. Results and Discussion
3.1. Characterization of Biochar and the Modified Biochar
3.2. Fourier Infrared Spectroscopy (FTIR) Analysis of Biochar Adsorption Strain JH1
3.3. Biomass Fixed by the Adsorption of Biochar
3.4. Effect of Water-Soluble Organic Carbon from Biochar
3.5. Adsorption and Degradation Kinetics of Phenol
3.6. Effect of Temperature, pH, Initial Phenol Concentration, and the Salinity
3.7. Effect of Recycling Biochar-Fixed Bacteria on Phenol Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Das, A.A.; Deka, D.; Naik, B.; Kumar Sahoo, N. Organic-inorganic hybrid hydroquinone bridged v-cds/hap/pd-tcpp: A novel visible light active photocatalyst for phenol degradation. J. Mol. Liq. 2021, 339, 116721. [Google Scholar] [CrossRef]
- Darbre, P.D. The history of endocrine-disrupting chemicals. Endocr. Metab. Res. 2019, 7, 26–33. [Google Scholar] [CrossRef]
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of phenolic compounds by basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Porębski, T.; Capała, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.Q. Biosorption of 2,4-dichlorophenol from aqueous solution by phanerochaete chrysosporium biomass: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2006, 137, 498–508. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Bioremediation of pahs and vocs: Advances in clay mineral–microbial interaction. Environ. Int. 2015, 85, 168–181. [Google Scholar] [CrossRef]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The immobilization of whole cells: Engineering principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Kim, P.; Johnson, A.M.; Essington, M.E.; Radosevich, M.; Kwon, W.-T.; Lee, S.-H.; Rials, T.G.; Labbé, N. Effect of pH on surface characteristics of switchgrass-derived biochars produced by fast pyrolysis. Chemosphere 2013, 90, 2623–2630. [Google Scholar] [CrossRef]
- Jin, Z.; Xiao, S.; Dong, H.; Xiao, J.; Tian, R.; Chen, J.; Li, Y.; Li, L. Adsorption and catalytic degradation of organic contaminants by biochar: Overlooked role of biochar’s particle size. J. Hazard. Mater. 2022, 422, 126928. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Girijan, S.; Kumar, M. Immobilized biomass systems: An approach for trace organics removal from wastewater and environmental remediation—sciencedirect. Curr Opin Environ Sci Health 2019, 12, 18–29. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghoshal, A.K. Biodegradation of real petroleum wastewater by immobilized hyper phenol-tolerant strains of Bacillus cereus in a fluidized bed bioreactor. 3 Biotech 2016, 6, 137. [Google Scholar] [CrossRef]
- Manya, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, H.; Xu, P.; Hou, B.; Jia, S.; Wang, D.; Li, K. Biodegradation of quinoline by streptomyces sp N01 immobilized on bamboo carbon supported Fe3O4 nanoparticles. Biochem. Eng. J. 2015, 99, 44–47. [Google Scholar] [CrossRef]
- Singh, N.; Balomajumder, C. Equilibrium isotherm and kinetic studies for the simultaneous removal of phenol and cyanide by use of S. Odorifera (Mtcc 5700) immobilized on coconut shell activated carbon. Appl. Water Sci. 2017, 7, 3241–3255. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Li, W.; Li, J.; Xia, D.; Xu, D.; Zhang, S.; Zhu, Y. Electron self-sufficient core-shell biocl@fe-biocl nanosheets boosting Fe(III)/Fe(II) recycling and synergetic photocatalysis-fenton for enhanced degradation of phenol. Appl. Catal. B Environ. 2023, 330, 122642. [Google Scholar] [CrossRef]
- Yang, H.J.; Yang, Z.M.; Xu, X.H.; Guo, R.B. Increasing the methane production rate of hydrogenotrophic methanogens using biochar as a biocarrier. Bioresour. Technol. 2020, 302, 122829. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Lew, B.; Cohen, E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Huang, Z.; Zhou, P.; Liu, S.-J. Degradation of aniline by newly isolated, extremely aniline-tolerant Delftia sp. AN3. Appl. Microbiol. Biotechnol. 2002, 58, 679–682. [Google Scholar] [CrossRef]
- Yu, J.; Tao, D.; Yu, S.; Kai, Y.; Wang, H. Biodegradation of phenol by entrapped cell of debaryomyces sp. With nano-Fe3O4 under hypersaline conditions. Int. Biodeterior. Biodegrad. 2017, 123, 37–45. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.-E.; Lee, S.S. Lead and copper immobilization in shooting range soil using soybean stover-and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Jin, X.; Liu, R.; Wang, H.; Han, L.; Qiu, M.; Hu, B. Functionalized porous nanoscale Fe3O4 particles supported biochar from peanut shell for pb(ii) ions removal from landscape wastewater. Environ. Sci. Pollut. Res. 2022, 29, 37159–37169. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, N.; Borham, A.; Zhang, S.; Xie, R.; Zhao, C.; Hu, J.; Wang, J. The effect of iron-modified biochar on phosphorus adsorption and the prospect of synergistic adsorption between biochar and iron-oxidizing bacteria: A review. Water 2023, 15, 3315. [Google Scholar] [CrossRef]
- Cheng, C.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Du, J.; Sun, P.; Feng, Z.; Zhang, X.; Zhao, Y. The biosorption capacity of biochar for 4-bromodiphengl ether: Study of its kinetics, mechanism, and use as a carrier for immobilized bacteria. Environ. Sci. Pollut. Res. 2016, 23, 3770–3780. [Google Scholar] [CrossRef]
- Liu, G.; Feng, K.; Cui, H.; Li, J.; Wang, M. Mof derived in-situ carbon- encapsulated fe3o4@c to mediate polysulfides redox for ultrastable lithium-sulfur batteries. Chem. Eng. J. 2019, 381, 122652. [Google Scholar] [CrossRef]
- Ramos-Escobedo, G.; Pecina-Treviño, E.; Tokunaga, A.B.; Concha-Guerrero, S.; Ramos-Lico, D.; Guerra-Balderrama, R.; Orrantia-Borunda, E. Bio-collector alternative for the recovery of organic matter in flotation processes. Fuel 2016, 176, 165–172. [Google Scholar] [CrossRef]
- Zhou, Y.; Petrova, S.P.; Edgar, K.J. Chemical synthesis of polysaccharide–protein and polysaccharide–peptide conjugates: A review. Carbohydr. Polym. 2021, 274, 118662. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; An, Q.; Zhou, Y.; Deng, S.; Miao, Y.; Zhao, B.; Yang, L. Highly synergistic effects on ammonium removal by the co-system of pseudomonas stutzeri xl-2 and modified walnut shell biochar. Bioresour. Technol. 2019, 280, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jian, Z.; Wang, Y.; Fang, C.; Hu, Q. Spatial–seasonal characteristics and influencing factors of dissolved organic carbon and chromophoric dissolved organic matter in poyang lake. Environ. Earth Sci. 2023, 82, 44. [Google Scholar] [CrossRef]
- Li, M.; Liu, M.; Joseph, S.; Jiang, C.; Wu, M.; Li, Z. Change in water extractable organic carbon and microbial plfas of biochar during incubation with an acidic paddy soil. Soil Res. 2015, 53, 763–771. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Shi, W.; Li, L.; Taherymoosavi, S.; Pan, G. Biochar DOM for plant promotion but not residual biochar for metal immobilization depended on pyrolysis temperature. Sci. Total Environ. 2019, 662, 571–580. [Google Scholar] [CrossRef]
- Jiang, Y.; Wen, J.; Bai, J.; Jia, X.; Hu, Z. Biodegradation of phenol at high initial concentration by alcaligenes faecalis. J. Hazard. Mater. 2007, 147, 672–676. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Thompson, M.L.; Lawrinenko, M. Characterization and quantification of biochar alkalinity. Chemosphere 2017, 167, 367–373. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of nitrate using paracoccus sp. Yf1 immobilized on bamboo carbon. J. Hazard. Mater. 2012, 229–230, 419–425. [Google Scholar] [CrossRef]
- Bugg, T.D.H. Dioxygenase enzymes: Catalytic mechanisms and chemical models. Tetrahedron 2003, 59, 7075–7101. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, J.; Hou, Y.; Li, H.; Du, C.; Jiang, B.; Shi, S. Effect of Fe3O4 nanoparticles exposure on the treatment efficiency of phenol wastewater and community shifts in sbr system. J. Hazard. Mater. 2021, 407, 124828. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Ni, J.; Sun, Y.; Xue, L.; Feng, Y.; Yu, Y.; Lin, X.; Yang, L. Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles. Chemosphere 2016, 147, 195–202. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhong, L.; Duan, J.; Feng, Y.; Yang, B.; Yang, L. Bioremediation of wastewater by iron oxide-biochar nanocomposites loaded with photosynthetic bacteria. Front. Microbiol. 2017, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Liu, M.; Bu, Y.; Zhang, J.; Chen, J.; Zhao, J. Adsorption–synergic biodegradation of diesel oil in synthetic seawater by acclimated strains immobilized on multifunctional materials. Mar. Pollut. Bull. 2015, 92, 195–200. [Google Scholar] [CrossRef]












| Samples | Yield (%) | Elemental Composition (%) | Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | O/C | H/C | (O + N)/C | ||
| Y3 | 44 | 59.44 | 5.19 | 32.70 | 2.54 | 0.13 | 0.550 | 0.087 | 0.593 |
| Y5 | 29.3 | 67.47 | 3.08 | 27.49 | 1.79 | 0.18 | 0.407 | 0.046 | 0.434 |
| Y7 | 25.1 | 67.51 | 1.71 | 29.18 | 1.43 | 0.17 | 0.432 | 0.025 | 0.453 |
| Fe-Y7 | — | 61.2 | 1.70 | 35.51 | 1.34 | 0.26 | 0.580 | 0.028 | 0.602 |
| Samples | Specific Surface Area (m2/g) | Pore Capacity (cm3/g) | Average Aperture (nm) |
|---|---|---|---|
| Y3 | 1.8796 | 0.003046 | 6.4819 |
| Y5 | 3.2917 | 0.005034 | 6.1169 |
| Y7 | 138.8126 | 0.070936 | 2.0441 |
| Fe-Y7 | 164.0975 | 0.097943 | 2.3874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Xiao, J.; Li, M.; Wu, J.; Zhang, T. Degradation of Phenol by Immobilized Alcaligenes faecalis Strain JH1 in Fe3O4-Modified Biochar from Pharmaceutical Residues. Water 2023, 15, 4084. https://doi.org/10.3390/w15234084
Zeng Z, Xiao J, Li M, Wu J, Zhang T. Degradation of Phenol by Immobilized Alcaligenes faecalis Strain JH1 in Fe3O4-Modified Biochar from Pharmaceutical Residues. Water. 2023; 15(23):4084. https://doi.org/10.3390/w15234084
Chicago/Turabian StyleZeng, Zhi, Jiahui Xiao, Manzhi Li, Jiahui Wu, and Taiping Zhang. 2023. "Degradation of Phenol by Immobilized Alcaligenes faecalis Strain JH1 in Fe3O4-Modified Biochar from Pharmaceutical Residues" Water 15, no. 23: 4084. https://doi.org/10.3390/w15234084