1. Introduction
In many countries, including the USA, agriculture and animal farming are the leading pollutant sources that significantly affect surface and groundwater quality. Over 50% of the total US population is dependent on surface water sources for drinking water [
1]. Agricultural and animal facility byproducts, such as nutrients, fertilizers, siltation, pesticides, metals, and pathogens originating from these operations, contaminate rivers, lakes, and reservoirs [
2]. Agricultural activities, specifically cropland irrigation and livestock production, have significantly increased and now account for 70% of all agricultural land. Other agricultural activities that cause water pollution in the US include poorly managed concentrated animal feeding operations (CAFOs), overgrazing, and overapplication of pesticides, irrigation water, and fertilizers [
3]. The United States Environmental Protection Agency (US EPA) has defined CAFOs as an agricultural operation that raises animals for at least 45 days per year, usually at high density, for the consumption of meat, eggs, or milk. In the US, the estimated annual manure produced by livestock is between three and 20 times more than what humans produce, which adds up to approximately two billion dry tons of manure every year [
4,
5].
In Missouri, there are 596 animal feeding operations, of which 502 are large enough to be classified as CAFOs. Approximately 2% of the state’s population lives within three miles of a CAFO, and approximately 15% of the state’s population lives within 10 miles of a CAFO [
6]. Major contaminants that can be found in CAFO wastes include organic matter, sediments, pathogens, veterinary pharmaceuticals, heavy metals, antibiotics, and naturally excreted hormones [
5].
Land application of stored CAFO manure is one of the most common disposal methods due to its relative ease and low cost. However, there are limits to this methodology because a given area of land can only handle a certain amount of nutrients from manure over a given time. The overapplication of CAFO wastes can overload soil with macronutrients (e.g., nitrogen and phosphorous) and micronutrients (e.g., heavy metals) that had been added to animal feed [
7]. In all cases, the wastes are directly applied onto agricultural land adjacent to or near the CAFO facilities. Only a few facilities attempt to reduce certain oxygen-demanding contaminants by aerating their lagoons because aeration is a relatively expensive treatment process [
8].
Pollutants enter surface water via either a point or nonpoint source. A point source can be a pipe or a drain that is often easily identifiable. Nonpoint sources occur over a wide area and are not easily attributed to a single source [
9]. The Clean Water Act defines CAFOs as point sources of pollution that are required to obtain permits to discharge waters into rivers and creeks used as drinking water [
10]. Regulatory enforcement and vested economic interest to adopt environmentally friendly production practices minimize agriculture’s contribution to water pollution [
11].
In 2014, the EPA concluded that, based on current technologies and associated costs, it is not practical for higher levels of wastewater treatment to be performed at typical farm operations. Therefore, the wastes from these operations will likely continue to be applied to land. Some of the components of that waste will find its way into surface water, and that water will be utilized for other agriculture needs and/or for human drinking water [
12].
The conventional process to remove turbidity, organic matter that includes synthetic organic compounds, and suspended solids from raw water sources include direct filtration, coagulation/settling treatment techniques, membrane-based systems, and absorption-based systems [
13,
14]. However, there is no single, most efficient and cost-effective process available for water treatment. Many drinking water treatment plants incorporate coagulation and flocculation processes by using alum- or polymer-based coagulants to remove turbidity, color, organic matter, and other contaminants [
15].
Over the last few decades, emerging nano-based techniques have created much interest in both the research and industrial communities and have also received the attention of drinking water treatment and wastewater treatment professionals. Researchers have primarily investigated the use of these methods for surface water treatment, such as at municipal drinking water treatment plants that may use river water as their source [
16,
17,
18]. Researchers have used magnetic nanoparticles alone to remove/adsorb various pollutants (e.g., pesticides, nitrates, and sulfates) from wastewater sources [
19,
20,
21]. Magnetic nanoparticle-based treatment technology has a major advantage in reducing the duration of the process as well as removing heavy metals and other pollutants. Applying an external magnetic field will help to overcome the shortcomings of nonmagnetic nanomaterials, which are difficult to isolate from the wastewater system [
22]. However, removing higher organic content and other pollutants when treating water from an industrial or agricultural source requires a coagulating agent. The combination of magnetic nanoparticles and a coagulating agent might be a beneficial alternative to the conventional method of water treatment as it would exhibit the floccule-forming capacity of coagulants [
23]. The use of magnetic nanoparticles has been favored for wastewater treatment due to their larger surface area, charge neutralization, and high absorption capability that can enhance coagulation/adsorption [
24]. Another advantage is their unique magnetic nature that leads to high activity and separation ease of flocs by faster separation/settling upon applying an external magnetic field [
25]. Magnetic nanoparticles functionalized with natural coagulant proteins have shown promising results in removing turbidity [
15,
26,
27].
The use of magnetic nanoparticles to purify wastewater systems has achieved great attention in recent years [
20,
28,
29,
30]. However, a literature review found no reports of using coagulants in combination with magnetic nanoparticles for CAFO wastewater treatment. Hence, this study investigated the potential of employing magnetic nanoparticles in combination with coagulants to assess water quality improvement. We hypothesized that water quality can be improved by magnetic nanosponges (MNSs) formed
in situ by mixing magnetic nanoparticles with coagulants in an optimal ratio. The total organic carbon (TOC), turbidity, total suspended solids (TSS), biological oxygen demand (BOD), and pH parameters were measured to establish the final water quality for consumer consumption based on EPA guidelines [
31]. Two magnetic nanoparticles, (i.e., commercial and in-house) along with conventional coagulants (i.e., polymer and aluminum) were used to treat CAFO wastewater collected in lagoons, and the water quality parameters were monitored. We evaluated the water quality improvement with the use of MNSs in removing impurities from the wastewater lagoons collected from dairy and swine farm CAFO facilities located at the University of Missouri. The comparison of various parameters as well as the pre- and post-treatments using MNSs are presented herein.
2. Materials and Methods
2.1. Materials
Ferrous chloride tetrahydrate (FeCl2·4H2O, ≥99.0%) and ferric chloride hexahydrate (FeCl3·6H2O, ≥98.0%) were procured from Millipore Sigma (St. Louis, MO, USA). Laboratory-grade hydrochloric acid (HCl) and sodium hydroxide (NaOH) were used as solvents for the synthesis process. Commercial iron oxide nanoparticles were purchased from Alpha Chemicals (Ward Hill, MA, USA). The commercial NPs, with an average particle size of 300 nm, were labelled “synthetic black iron oxide”. They were stored at room temperature in the original packet and used as such.
Alum-based coagulant (BASF AH-9937) and polymer-based coagulants (BASF Zetag 8814, 8816, 8818, 8819, and 8846-FS) were provided by the BASF chemical dealer located in Columbia, MO (USA). Coagulants were diluted to prepare a stock solution of 2% and stored at room temperature until they were used.
2.2. Concentrated Animal Feeding Operations Facilities
Two CAFO lagoons (i.e., dairy and swine farms) were chosen for wastewater collection. The dairy facility is located at the Foremost Dairy Research Center (Columbia, MO, USA), and the swine facility is located at the South Farm Research Center (Columbia, MO, USA), both of which are part of the University of Missouri. The CAFOs do not have an onsite treatment plant. The wastewater from these lagoons is concentrated during the summer, and once each year, the highly concentrated lagoon water is applied to the cattle grazing area and agricultural fields.
For each experiment, about 15 L of lagoon water was collected during the morning. The same sample collection site was used at each CAFO. At the dairy farm, the reused lagoon water was collected in a plastic container before it reached the lagoon, whereas at the swine farm, the sample water was collected from a particular area near the source that was easily accessible by foot. The collected wastewater was filtered through a cheesecloth to remove any wooden pieces, animal waste, and insects, and it was properly mixed in a container before loading it into the testing jars.
2.3. Instrumentation
The portable turbidimeter (HACH, Loveland, CO, USA) with a range of 0 to 1000 NTU was used to measure the turbidity. A TOC-VW analyzer from Shimadzu (Kyoto, Japan) was used to determine the total organic content (TOC) of the water sample that contained an inherited automatic acid addition to remove the inorganic carbon content from the sample. Analyses of the biological oxygen demand (BOD) and total suspended solids (TSS) were performed at Engineering Surveys & Services, Columbia, MO, USA. The Zetasizer (Nano ZS from Malvern Panalytical, Malvern, UK), which employs a dynamic light scattering (DLS) technique, was used to measure the hydrodynamic size and zeta potential of the synthesized nanoparticles as well as the commercial nanoparticles. Transmission electron microscopy (TEM, Columbia, MO, USA) was used to image and confirm the core size of the nanoparticles.
2.4. Synthesis of Magnetic Nanoparticles (MNPs)
Two different magnetic nanoparticles were used in the present study. Commercially produced magnetic nanoparticles (CMNPs) were used for the experiments. Sphere- shaped magnetic nanoparticles (SMNPs) were produced following a modified reported procedure [
32]. The MNPs were synthesized in an inert condition. First, 25 mL of water was deoxygenated by bubbling nitrogen gas for 30 min, after which 0.85 mL of 12.1 N HCl was added, and the bubbling was continued for another 10 min. Upon completion of the deoxygenation, 2.0 g of ferrous chloride tetrahydrate (FeCl
2·4H
2O; 0.01 mol) and 5.2 g of ferric chloride hexahydrate (FeCl
3·6H
2O; 0.02 mol) were added and gently stirred for 1 min until the solution was completely mixed. The resultant iron oxide solution was added dropwise to 250 mL of 1.5 M NaOH with vigorous stirring until a black precipitate was formed. The suspension was then sonicated for 10 min, and nitrogen gas bubbled on the headspace above the solution with the stopper installed. The presence of MNSs were validated by confirming their magnetic attraction towards a N50-grade neodymium block magnet with a pull force of 250 lb [
33]. The suspension was stored at room temperature, and 1 mL was centrifuged at 20,000×
g for 20 min and vacuum-dried for 4 h to determine the concentration (mg/mL). The isolated magnetic nanoparticles were characterized by their standard hydrodynamic size and zeta potential measurements.
2.5. Flocculation Tests
Varying concentrations of coagulants were added to 200 mL of crude water samples collected from the CAFO lagoons, and samples were “tossed” from one jar to another for a total of 10 tosses. The samples were observed during the tosses to determine when flocs were created and further observed to see if the flocs held together (remained intact) or fell apart (the flocs did not remain intact). Initially, toss testing was started with a lower (50 ppm) concentration of coagulants. During the process, it was increased to 400 ppm to determine the optimal concentration of coagulant that can form and hold the flocs.
2.6. Water Purification
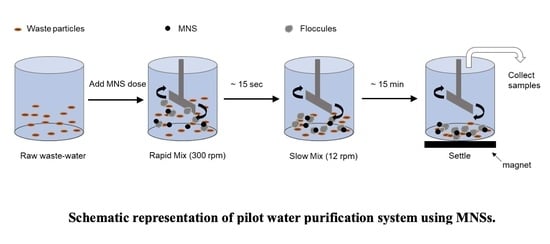
The pilot water purification system consisted of a plastic jar with six individual 2 L compartments (
Figure 1). The jars were filled with 2 L of sample water and placed on the tester, after which stir paddles were inserted and operated at a mixing speed of 300 rpm. The optimal concentration of coagulants and MNPs were mixed at ratios of 0, 10, 25, 75, and 100% to form MNSs and rapidly stirred at 300 rpm for 15 sec to stimulate rapid mixing. The mixing paddles were then slowed to 12 rpm and operated at this slower speed for 15 min to simulate a slow mix. After the slow mixing was completed, the stirrer was turned off, and the paddles were removed. The samples were placed on a set of six disk magnets, the water was allowed to settle, and the time was measured. Purified supernatant water samples were collected at three settling time points (i.e., 5, 10, and 20 min) and used for the TOC, turbidity, BOD, and TSS measurements. The data presented is an average of the three measurements performed for each sample.
Water Quality Parameters: The four well-established parameters of water quality, turbidity, total organic carbon content (TOC), biological oxygen demand (BOD), and total suspended solids (TSS), were determined beore and after the wastewater treatment, and the percentage of removal was calculated using the following equation:
3. Results and Discussion
3.1. Synthesis and Characterization of Magnetic Nanoparticles
Two magnetic nanoparticles (MNPs) were used for the present study, with one (CMNP) from a commercial source and the other (SMNP) synthesized in our laboratories. The CMNP was purchased and used with no modification while the SMNP was synthesized following a slightly modified reported procedure [
32]. Typically, the synthesis process involves the precipitation of an iron chloride solution using sodium hydroxide as the precipitant (
Figure 2). The SMNPs, obtained as a black precipitate, were stored as a suspension in water whereas the commercial MNPs were stored in their original powder form. MNPs were characterized by TEM, zeta potential, and hydrodynamic size measurements (
Table 1). TEM images recorded for these particles indicated that the SMNPs were smaller (<20 nm) than the CMNPs (>200 nm). The morphology of the SMNPs was spherical with slight irregularities while the CMNPs had defined edges, including rhombic shapes (
Figure 3). The particle clustering seen in the images are a result of air-drying the sample as well as the magnetic attraction of particles. Additionally, while the SMNPs showed uniformly independent nanoparticles, individual CMNPs were coated with smaller-shaped particles. The hydrodynamic diameter of the SMNPs was smaller than the CMNPs. The zeta potential of the SMNPs was more negative compared to the commercial MNPs. The higher shift in negative zeta potential value of the SMNPs suggests greater stability compared to the CMNPs [
34]. Nanoparticles with a higher zeta potential are more stable because they have highly charged particles, which lead to electric repulsion and prevent the aggregation of particles [
35]. The magnetic properties of the SMNPs and CMNPs were confirmed by placing the precipitate on a magnet.
3.2. Synthesis of Magnetic Nanosponges
Magnetic nanosponges (MNSs) were prepared in situ using a constant amount of coagulants and varying concentrations of both magnetic nanoparticles (i.e., CMNPs and SMNPs). Coagulants were chosen based on the flocculation test. The coagulant concentration was kept constant while the MNP concentration was varied.
3.3. Choice and Optimization of Coagulant Concentration
A toss-testing flocculation study was conducted to choose the coagulants. Flocculation studies were conducted using one aluminum (BASF AH-9937) and five polymer-based coagulants (BASF Zetag 8814, 8816, 8818, 8819, and 8846-FS) in the raw water collected from the dairy lagoon. The toss test was conducted using different coagulants of varying concentrations. Toss-testing for each coagulant was started at a concentration of 50 ppm and gradually increased until the optimal flocculation was obtained. Coagulants were mixed with raw water samples and were “tossed” from one jar to another for 10 total tosses. The samples were observed during the tosses to determine when flocs were created and if the flocs held together or fell apart. If the flocs fell apart, the coagulant was rejected. In general, polymer-based coagulants formed flocs immediately, but the aluminum coagulant did not. Among the five polymer-based coagulants, Zetag 8814 and 8816 were the ones that formed floccules and held together while the remaining either could not form floccules or yield clear (or somewhat clear) liquid after decantation. Therefore, these two polymer coagulants were chosen for purification (
Table S1).
The levels of turbidity and contaminants varied depending upon the CAFO. Therefore, the optimal amount of pure coagulant required to form a floc and remove contaminants with no residual amounts had to be determined for each CAFO. The lagoon water was treated with varying concentrations of coagulants, and the optimal concentration of the coagulants was determined from the TOC and turbidity measurements. The TOC and turbidity measurements were performed following treatments with different coagulant concentrations. The coagulant concentration that resulted in the lowest TOC and turbidity levels for raw water was chosen as optimal. Based on the toss studies, the optimal coagulant concentration for swine water was 20 ppm while for the highly concentrated dairy lagoon water, the optimal concentration was 400 ppm. These optimal concentrations of coagulants were used to form MNSs with varying ratios of MNPs.
3.4. Optimization of MNPs for CAFO Water Purification
Magnetic nanosponges (MNSs) were prepared in situ by mixing coagulants and MNPs. The optimal ratio of MNPs to coagulant required to form MNSs that could effectively remove the contaminants was determined by performing a pilot experiment using the dairy lagoon water. The optimal coagulant concentration (400 ppm), as established by the flocculation experiment, was used, and the ratio of MNPs was varied. The coagulant concentration was kept constant while the ratio of the MNP to coagulant was varied from 0–100% to produce the MNSs. This means that the 0% solution had no MNPs but only coagulant whereas the 100% MNP solution had no coagulant but only MNPs. The various ratios of MNPs (i.e., 0, 10, 25, 50, 75, and 100%) in the coagulant mixture were added to six different jars that contained 2 L of water collected from the dairy lagoon. These jars were stirred, and the contaminants were allowed to settle using a magnet. The sedimentation time was varied from 5 to 20 min at 5 min intervals, and the supernatant water was collected. The turbidity and TOC values of the supernatant water were compared pre- and posttreatment with the MNSs. The initial results obtained from the dairy water confirmed that the best purification was achieved at a 75% ratio of MNP to coagulant at all time points, so the same ratio was used for the remaining studies.
3.5. Dairy Farm Water
The efficiency of the magnetic nanosponges to purify the dairy farm water was tested using both polymer coagulants (Zetag 8814 and 8816) along with both MNPs. The turbidity and TOC values of the raw water were measured prior to the formation of the MNSs and used as controls. The percentage reduction in turbidity and TOC for varying ratios of the two MNPs with coagulants at different settling time points was calculated. Raw water from the dairy farm lagoon was so highly concentrated and turbid that the values were out of the range of the turbidimeter, which can only measure up to 1000 NTU. However, upon the addition of the coagulants, the turbidity decreased, and a detectable value of ~570 NTU was observed. Additional turbidity reduction was obtained by using coagulants along with MNPs. At all of the time points, the turbidity reduction was six- and fifteen-fold upon increasing the ratio of the CMNPs and SMNPs to the coagulants, respectively (
Table S2). The TOC of the raw water was 500 ppm. Upon coagulant addition, a 10% reduction was achieved at 5 min while the reduction was 20% at 20 min. Compared to pure coagulants, an additional twofold decrease was achieved upon the addition of a 75% ratio of MNPs to coagulants at a 20 min settling time.
A gradual decrease in turbidity (~2-fold) and TOC (~1.6-fold) was observed upon increasing the ratio of both the MNPs to coagulants from 5% to 75% whereas the settling time did not significantly influence the results. The maximum turbidity and TOC reduction were achieved with a 75% ratio of MNPs to coagulants at a 20 min settling time (
Figure 4).
Pristine MNPs were not effective at removing turbidity and TOC. The turbidity and TOC reduction were attributed to the formation of MNSs. The MNSs formed with the SMNP were more effective in reducing the turbidity compared to those formed with the CMNP. There was a minimal difference between the two coagulants in forming MNSs and in the percentage of turbidity reduction at different ratios at all time points. There was no difference between the performance of the MNSs formed with the SMNP and CMNP in reducing the TOC.
3.6. Swine Farm Water
The efficiency of the magnetic nanosponges to purify swine farm water was tested with polymer coagulants (Zetag 8814 and 8816) along with both MNPs. In addition to the turbidity and TOC, TSS and BOD values were measured for both the raw and treated water. Based on the results of the dairy farm water, MNSs showed the best performance at a 75% ratio of MNPs to coagulants. Therefore, only three ratios (i.e., 0, 75, and 100%) were used for testing. MNSs were formed
in situ at a ratio of 75% MNPs to coagulants. The coagulant concentration was maintained at 20 ppm. The turbidity, TOC, BOD, and TSS values obtained for the treated water samples and collected at 5 min and 20 min settling time points are listed in
Table S3.
The percentage of reduction in turbidity, TOC, BOD, and TSS was calculated for the water samples collected at 5 min and 20 min settling time points. The pristine coagulants Zetag 8814 and 8816 were effective in reducing the turbidity by five- and three-fold, respectively. An additional reduction of 5% and 14% was achieved upon the formation of MNSs with Zetag 8814 and Zetag 8816 coagulants, respectively. The use of different MNPs in the formation of the MNSs had minimal or no influence on the turbidity reduction. The TOC reduction was minimal (~1.2-fold) with the use of pure coagulants. A reduction of up to 10% was achieved upon the formation of MNSs with a 75% ratio of MNPs to coagulants. However, the MNSs formed with a 75% ratio of SMNP to Zetag 8814 were only able to reduce the TOC up to 4% compared to the 10% reduction achieved with Zetag 8816. The pure coagulants Zetag 8814 and 8816 were effective in reducing the BOD twofold. An additional reduction of up to 10% and 35% was achieved upon the formation of the MNSs with the Zetag 8814 and Zetag 8816 coagulants, respectively, with both MNPs. The pure coagulants Zetag 8814 and 8816 were effective in reducing the TSS twofold. An additional reduction up to 26% and 19% was achieved upon the formation of MNSs with a 75% ratio of SMNPs and CMNPs to coagulants, respectively (
Figure 5).
3.7. Discussion
The use of MNPs along with coagulants to remove turbidity has been investigated by a limited number of researchers. There is evidence that the combination of MNPs and natural coagulants (i.e., proteins from
Moringa oleifera seeds) with sedimentation under the influence of an external magnetic field can reduce settling times, result in a more compacted sludge, and increase the turbidity removal efficiency [
15,
23,
24,
25,
26,
27]. Mateus et al. [
15] reported that magnetic nanoparticles functionalized with proteins from
Moringa oleifera seeds showed 96.8% efficiency (143.33 NTU) in turbidity removal after 10 min of magnetic sedimentation in raw water collected from a Brazilian river basin. However, in this study, functionalized nanoparticles with natural coagulants were applied to the coagulation/flocculation and sedimentation (CFS) process of superficial raw water under the influence of an external magnetic field. Triques et al. [
23] performed a similar study wherein they treated an effluent from the cleaning-in-place (CIP) of a dairy industry with iron oxide nanoparticles functionalized with the Moringa seed extract and achieved about 90% turbidity removal with a reduced sedimentation time (i.e., 7 min compared to 60 min when
Moringa oleifera seed extract alone was used). The present study used two types of magnetic particles along with polymer coagulants that are routinely used for water purification. MNPs alone were not effective in removing turbidity, TOC, BOD, and TSS whereas they were effective after being combined with coagulants. MNSs significantly improved the quality of water by reducing all four parameters. The highest reduction occurred in the turbidity, with a moderate reduction for the TSS and BOD and a minimal reduction for the TOC. However, the extent of the reduction in these parameters varied depending upon the type of coagulants and MNPs used in the formation of the MNSs. The use of different MNPs in the formation of the MNSs had minimal or no influence on the turbidity reduction whereas MNSs formed with Zetag 8814 along with CMNPs were better at the TOC removal compared to those formed with SMNPs. MNSs formed with Zetag 8816, combined with each of the two MNPs, were superior in reducing the BOD compared to Zetag 8814. The use of different MNPs in the formation of MNSs had no influence in the BOD reduction. MNSs formed with SMNPs and coagulants were more effective at the TSS reduction compared to MNSs formed with CMNPs. Increasing the settling time had no influence on the parameters.