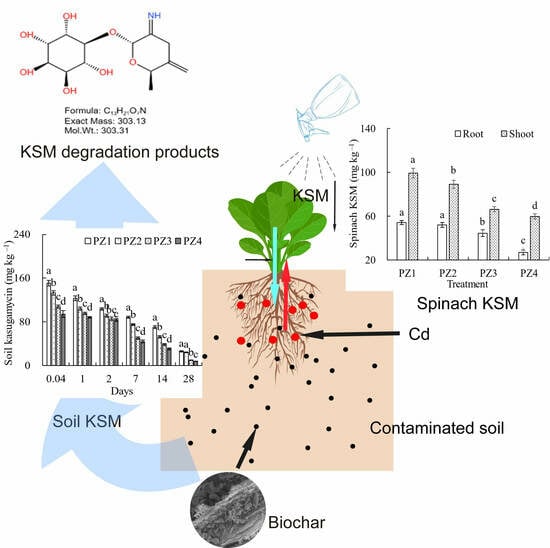
Biochar and Cd Alter the Degradation and Transport of Kasugamycin in Soil and Spinach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. KSM Extraction and Chromatographic Analysis Methods
2.3. Functional-Group Characterization of Biochar and Soil
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of Biochar on pH and SOC
3.2. Effects of Biochar on KSM Distribution in Soil and Spinach
3.3. KSM and Degradation Products
3.4. Functional-Group Characteristics of Raw Biochar and Soil
3.5. The Relationship between KSM, Biochar, Soil Cd, and Soil Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maggi, F.; Tang, F.H.M.; Black, A.J.; Marks, G.B.; McBratney, A. The pesticide health risk index—An application to the world’s countries. Sci. Total Environ. 2021, 801, 149731. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT. In Database Collection of the Food and Agriculture Organization of the United Nations 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Available online: https://www.fao.org/3/cb6034en/cb6034en.pdf (accessed on 12 December 2022).
- Liang, Y.; Duan, Y.; Fan, C.; Dong, H.; Yang, J.; Tang, J.; Tang, G.; Wang, W.; Jiang, N.; Cao, Y. Preparation of kasugamycin conjugation based on ZnO quantum dots for improving its effective utilization. Chem. Eng. J. 2019, 361, 671–679. [Google Scholar] [CrossRef]
- Kasuga, K.; Sasaki, A.; Matsuo, T.; Yamamoto, C.; Minato, Y.; Kuwahara, N.; Fujii, C.; Kobayashi, M.; Agematu, H.; Tamura, T.; et al. Heterologous production of kasugamycin, an aminoglycoside antibiotic from Streptomyces kasugaensis, in Streptomyces lividans and Rhodococcus erythropolis L-88 by constitutive expression of the biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2017, 101, 4259–4268. [Google Scholar] [CrossRef]
- Zhang, Y.; Aleksashin, N.A.; Klepacki, D.; Anderson, C.; Vázquez-Laslop, N.; Gross, C.A.; Mankin, A.S. The context of the ribosome binding site in mRNAs defines specificity of action of kasugamycin, an inhibitor of translation initiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2118553119. [Google Scholar] [CrossRef] [PubMed]
- Rattinam, R.; Basha, R.S.; Wang, Y.L.; Wang, Z.C.; Hsu, N.S.; Lin, K.H.; Zadeh, S.M.; Adhikari, K.; Lin, J.P.; Li, T.L. KasQ an Epimerase Primes the Biosynthesis of Aminoglycoside Antibiotic Kasugamycin and KasF/H Acetyltransferases Inactivate Its Activity. Biomedicines 2022, 10, 212. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, S.; Deng, L.; Chen, Y.; Liu, X.; Li, D. Residues and Dynamics of Kasugamycin in Chilli and Soil. Bull. Environ. Contam. Toxicol. 2012, 89, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Alechaga, E.; Moyano, E.; Teresa Galceran, M. Simultaneous analysis of kasugamycin and streptomycin in vegetables by liquid chromatography-tandem mass spectrometry. Anal. Methods 2015, 7, 3600–3607. [Google Scholar] [CrossRef]
- Jurgens, A.G.; Babadoost, M. Sensitivity of Erwinia amylovora in Illinois Apple Orchards to Streptomycin, Oxytetracyline, Kasugamycin, and Copper. Plant Dis. 2013, 97, 1484–1490. [Google Scholar] [CrossRef]
- Chen, G.; Qiao, Y.; Liu, F.; Zhang, X.; Liao, H.; Zhang, R.; Dong, J. Dissipation and dietary risk assessment of kasugamycin and saisentong in Chinese cabbage. Environ. Sci. Pollut. Res. 2020, 27, 35228–35238. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ho, C.-H.; Lin, C.-J.; Lo, C.-C. Exposure effect of fungicide kasugamycin on bacterial community in natural river sediment. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2010, 45, 485–491. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Xiang, X.; Zhang, Q.; Zhao, W.; Wei, G.; Hu, A. Uptake and transport of antibiotic kasugamycin in castor bean (Ricinus communis L.) seedlings. Front. Microbiol. 2022, 13, 948171. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wen, Y.; Yang, W.; Zhang, X.; Xia, M.; Zhou, N.; Xiong, Y.; Zhou, Z. The mechanism transformation of ramie biochar’s cadmium adsorption by aging. Bioresour. Technol. 2021, 330, 124947. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xiao, H.; Zhang, J.B.; Dai, C.; Li, T.; Gao, M.-t.; Hu, J.; Li, J. Characterization of highly stable biochar and its application for removal of phenol. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef] [PubMed]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.G.; Ippolito, J.A.; Rinklebe, J. Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.A.; Rathke, S.J.; Karlen, D.L. Biochar impact on Midwestern Mollisols and maize nutrient availability. Geoderma 2014, 230, 340–347. [Google Scholar] [CrossRef]
- Varjani, S.; Kumar, G.; Rene, E.R. Developments in biochar application for pesticide remediation: Current knowledge and future research directions. J. Environ. Manag. 2019, 232, 505–513. [Google Scholar] [CrossRef]
- Ogura, A.P.; Lima, J.Z.; Marques, J.P.; Massaro Sousa, L.; Rodrigues, V.G.S.; Espíndola, E.L.G. A review of pesticides sorption in biochar from maize, rice, and wheat residues: Current status and challenges for soil application. J. Environ. Manag. 2021, 300, 113753. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Asif Naeem, M.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef]
- Jones, D.L.; Edwards-Jones, G.; Murphy, D.V. Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol. Biochem. 2011, 43, 804–813. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, G. Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ. Sci. Technol. 2003, 37, 3635–3639. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Abas, K.; Brisson, J.; Amyot, M.; Brodeur, J.; Storck, V.; Montiel-León, J.M.; Duy, S.V.; Sauvé, S.; Kõiv-Vainik, M. Effects of plants and biochar on the performance of treatment wetlands for removal of the pesticide chlorantraniliprole from agricultural runoff. Ecol. Eng. 2022, 175, 106477. [Google Scholar] [CrossRef]
- Cui, L.; Yin, C.; Chen, T.; Quan, G.; Ippolito, J.A.; Liu, B.; Yan, J.; Hussain, Q. Biochar Immobilizes and Degrades 2,4,6-Trichlorophenol in Soils. Environ. Toxicol. Chem. 2019, 38, 1364–1371. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Q.; Ma, J.; Cui, L.; Quan, G.; Yan, J.; Wu, L.; Hina, K.; Abdul, B.; Wang, H. Effects of biochar on cadmium (Cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ. Pollut. Bioavailab. 2020, 32, 36–46. [Google Scholar] [CrossRef]
- Li, Z.; Qi, X.; Fan, X.; Du, Z.; Hu, C.; Zhao, Z.; Isa, Y.; Liu, Y. Amending the seedling bed of eggplant with biochar can further immobilize Cd in contaminated soils. Sci. Total Environ. 2016, 572, 626–633. [Google Scholar] [CrossRef]
- Yang, D.; Yang, S.; Wang, L.; Xu, J.; Liu, X. Performance of biochar-supported nanoscale zero-valent iron for cadmium and arsenic co-contaminated soil remediation: Insights on availability, bioaccumulation and health risk. Environ. Pollut. 2021, 290, 118054. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Huang, Q.; Li, B.; Ma, T.; Qin, X.; Zhao, L.; Sun, Y.; Xu, Y. Effects of sepiolite and biochar on the photosynthetic and antioxidant systems of pakchoi under Cd and atrazine stress. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2022, 57, 897–904. [Google Scholar] [CrossRef]
- Gong, Z.T.; Lei, W.J.; Chen, Z.C.; Gao, Y.X.; Zeng, S.G.; Zhang, G.L.; Xiao, D.N.; Li, S.G. Chinese Soil Taxonomy; Science Press: Beijing, China, 2001. [Google Scholar]
- Lu, R. Methods of inorganic pollutants analysis. In Soil and Agro-Chemical Analysis Methods; China Agriculture Science and Technique Press: Beijing, China, 2000; pp. 205–266. [Google Scholar]
- IBI. Standardized product definition and product testing guidelines for biochar that is used in soil. In IBI Biochar Standards; IBI: Toronto, ON, Canada, 2012. [Google Scholar]
- Zhang, Q.; Zhou, Y.; Tang, L.; Zhang, N.; Zhang, Z. Residue and decline study of kasugamycin in paddy water and paddy soil. Chin. J. Pestic. Sci. 2012, 14, 533–538. [Google Scholar]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, J.X.; Kinder, C.E.S.; Yang, X.; Slany, M.; Wang, B.; Song, H.; Shaheen, S.M.; Leinweber, P.; Rinklebe, J. Rice hull biochar enhances the mobilization and methylation of mercury in a soil under changing redox conditions: Implication for Hg risks management in paddy fields. Environ. Int. 2022, 168, 107484. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Liu, B.L.; Cheng, K.; Antonietti, M.; Yang, F. Moderating carbon dynamics in black soil by combined application of biochar and an artificial humic substance. Land Degrad. Dev. 2023, 34, 1352–1362. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Y.; Yan, J.; Hina, K.; Hussain, Q.; Qiu, T.; Zhu, J. Revitalizing coastal saline-alkali soil with biochar application for improved crop growth. Ecol. Eng. 2022, 179, 106594. [Google Scholar] [CrossRef]
- You, X.; Suo, F.; Yin, S.; Wang, X.; Zheng, H.; Fang, S.; Zhang, C.; Li, F.; Li, Y. Biochar decreased enantioselective uptake of chiral pesticide metalaxyl by lettuce and shifted bacterial community in agricultural soil. J. Hazard. Mater. 2021, 417, 126047. [Google Scholar] [CrossRef]
- Awan, S.; Ippolito, J.A.; Ullman, J.L.; Ansari, K.; Cui, L.; Siyal, A.A. Biochars reduce irrigation water sodium adsorption ratio. Biochar 2021, 3, 77–87. [Google Scholar] [CrossRef]
- Alfonso, R.-V. Changes on the Phytoavailability of Nutrients in a Mine Soil Reclaimed with Compost and Biochar. Water Air Soil Pollut. 2016, 227, 453. [Google Scholar] [CrossRef]
- Gao, W.K.; Yang, Y.Y.; Zong, H.Y.; Liu, J.; Song, N.N.; Wang, F.L. Simultaneously Sorptive Reduction in Cadmium and Glyphosate Diffuse loss by Biochar-Amended Soil. Fresenius Environ. Bull. 2020, 29, 4545–4555. [Google Scholar]
- Li, R.; Wen, Y.; Liu, M.; Su, L.; Wang, Y.; Li, S.; Zhong, M.-e.; Zhou, Z.; Zhou, N. Simultaneous removal of organic inorganic composite contaminants by in situ double modified biochar: Performance and mechanisms. J. Taiwan Inst. Chem. Eng. 2022, 139, 104523. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Ying, G.-G.; Kookana, R.S. Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 2009, 76, 665–671. [Google Scholar] [CrossRef]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a Promising Strategy for Pesticide-Contaminated Soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Slack, S.M.; Walters, K.J.; Outwater, C.A.; Sundin, G.W. Effect of Kasugamycin, Oxytetracycline, and Streptomycin on In-orchard Population Dynamics of Erwinia amylovora on Apple Flower Stigmas. Plant Dis. 2021, 105, 1843–1850. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Borchard, N.; Zajkoska, P.; Fernández-Bayo, J.D.; Martinazzo, R.; Berns, A.E.; Burauel, P. Biochar-Mediated [14C]Atrazine Mineralization in Atrazine-Adapted Soils from Belgium and Brazil. J. Agric. Food Chem. 2013, 61, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Pang, H.; Zhou, Z.; Zhang, P.; Feng, Y.; Sheng, G.D. Competitive biodegradation of dichlobenil and atrazine coexisting in soil amended with a char and citrate. Environ. Pollut. 2009, 157, 2964–2969. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Fan, Q.; Sun, J.; Quan, G.; Yan, J.; Hina, K.; Wang, H.; Zhang, Z.; Hussain, Q. Changes in surface characteristics and adsorption properties of 2,4,6-trichlorophenol following Fenton-like aging of biochar. Sci. Rep. 2021, 11, 4293. [Google Scholar] [CrossRef]
- Cui, L.; Chen, T.; Quan, G.; Xiao, B.; Ma, Y.; Pan, M.; Liu, Y.; Liu, B.; Yin, C.; Yan, J.; et al. Renewable Material-derived Biochars for the Efficient Removal of 2,4-Dichlorophen from Aqueous Solution: Adsorption/Desorption Mechanisms. Bioresources 2017, 12, 4912–4925. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Cares, X.A.; Pérez, M.A.; del Pino, J.N. Biochar increases pesticide-detoxifying carboxylesterases along earthworm burrows. Sci. Total Environ. 2019, 667, 761–768. [Google Scholar] [CrossRef]
- Zhang, S.; Hua, Z.; Yao, W.; Lü, T.; Chen, Y.; Fang, Z.; Zhao, H. Use of corn straw-derived biochar for magnetic solid-phase microextraction of organophosphorus pesticides from environmental samples. J. Chromatogr. A 2021, 1660, 462673. [Google Scholar] [CrossRef]
- Mazarji, M.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Barakhov, A.; Barbashev, A.; Dudnikova, T.; Lobzenko, I.; Giannakis, S. Decrypting the synergistic action of the Fenton process and biochar addition for sustainable remediation of real technogenic soil from PAHs and heavy metals. Environ. Pollut. 2022, 303, 119096. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Zhou, Q.; Sun, Z.; Ma, Z. Sewage sludge derived biochars provoke negative effects on wheat growth related to the PTEs. Biochem. Eng. J. 2019, 152, 107386. [Google Scholar] [CrossRef]





| pH | CEC (cmol kg−1) | EC (dS m−1) | OC (g kg−1) | Total N (g kg−1) | Total P (g kg−1) | Total K (g kg−1) | Ash (%) | |
|---|---|---|---|---|---|---|---|---|
| Soil | 8.7 | 2.4 | 4.1 | 6.5 | 0.7 | 0.4 | 7.3 | NA |
| Biochar | 10.4 | 10.6 | 4.4 | 650.2 | 6.3 | 20.4 | 16.9 | 28.6 |
| Treatment | Shoot Biomass | Root Biomass |
|---|---|---|
| (g box−1) | ||
| PZ1 | 76.7 ± 1.2 b | 11.8 ± 1.0 ab |
| PZ2 | 74.0 ±2.3 b | 10.7 ± 0.8 b |
| PZ3 | 108.9 ± 4.2 a | 14.7 ± 2.2 a |
| PZ4 | 117.3 ± 5.6 a | 14.9 ± 3.4 a |
| C14H25O9N3 | C14H29O8N3 | C14H27O7N3 | C12H24O8N2 | C13H21O7N | C8H17O8N |
| C8H13O4N3 | C7H13ON3 | C7H14ON2 | C6H14O2N2 | C6H10O | C3H9N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Ma, J.; Quan, G.; Yan, J.; Ippolito, J.A.; Wang, H. Biochar and Cd Alter the Degradation and Transport of Kasugamycin in Soil and Spinach. Agriculture 2023, 13, 2172. https://doi.org/10.3390/agriculture13112172
Cui L, Ma J, Quan G, Yan J, Ippolito JA, Wang H. Biochar and Cd Alter the Degradation and Transport of Kasugamycin in Soil and Spinach. Agriculture. 2023; 13(11):2172. https://doi.org/10.3390/agriculture13112172
Chicago/Turabian StyleCui, Liqiang, Jingwen Ma, Guixiang Quan, Jinlong Yan, James A. Ippolito, and Hui Wang. 2023. "Biochar and Cd Alter the Degradation and Transport of Kasugamycin in Soil and Spinach" Agriculture 13, no. 11: 2172. https://doi.org/10.3390/agriculture13112172