EEG Feature Analysis Related to Situation Awareness Assessment and Discrimination
Abstract
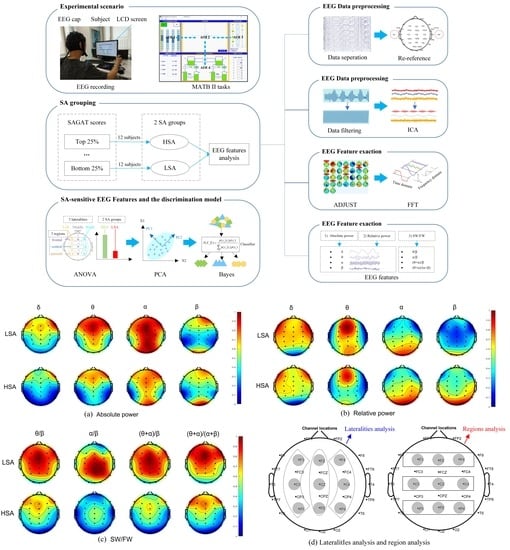
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Subjects
2.3. Experimental Platform and Tasks
2.4. EEG Equipment and Indicators
2.5. EEG Equipment and Indicators
3. Results and Analysis
3.1. Grouping of SA Levels
3.2. Quantitative EEG Analysis of Different SA Groups
3.2.1. Data Analysis Methods
3.2.2. Absolute Power of Different SA Groups
3.2.3. Relative Power of Different SA Groups
3.2.4. SW/FW Ratios of Different SA Groups
3.3. Bayesian Discrimination Model of SA Based on Sensitive EEG Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.; Zhang, R.; Yin, Z.; Li, Z. Human Errors and Human Reliability. In Handbook of Human Factors and Ergonomics; Salvendy, G., Karwowski, W., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 514–572. ISBN 978-1-119-63608-3. [Google Scholar]
- Endsley, M.R.; Jones, D.G. Designing for Situation Awareness: An Approach to User-Centered Design, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Endsley, M.R. Toward a Theory of Situation Awareness in Dynamic Systems. Hum. Factors 1995, 37, 32–64. [Google Scholar] [CrossRef]
- Stanton, N.A.; Salmon, P.M.; Walker, G.H.; Salas, E.; Hancock, P.A. State-of-Science: Situation Awareness in Individuals, Teams and Systems. Ergonomics 2017, 60, 449–466. [Google Scholar] [CrossRef]
- Nguyen, T.; Lim, C.P.; Nguyen, N.D.; Gordon-Brown, L.; Nahavandi, S. A Review of Situation Awareness Assessment Approaches in Aviation Environments. IEEE Syst. J. 2019, 13, 3590–3603. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, R.; Lin, S.; Schultz, M.; Delahaye, D. The Impact of Automation on Air Traffic Controller’s Behaviors. Aerospace 2021, 8, 260. [Google Scholar] [CrossRef]
- Taylor, R.M. Situation Awareness Rating Technique (SART): The Development of a Tool for Aircrew Systems Design; Advisory Group for Aerospace Research & Development: Neuilly Sur Seine, France, 1990; pp. 3-1–3-17. [Google Scholar]
- Valerie Jane, G. Measures of Situational Awareness. In Human Performance and Situation Awareness Measures; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–174. ISBN 978-0-429-00102-4. [Google Scholar]
- Durso, F.T.; Hackworth, C.A.; Truitt, T.R.; Crutchfield, J.; Nikolic, D.; Manning, C.A. Situation Awareness as a Predictor of Performance for En Route Air Traffic Controllers. Air Traffic Control Q. 1998, 6, 1–20. [Google Scholar] [CrossRef]
- Endsley, M.R. Measurement of Situation Awareness in Dynamic Systems. Hum. Factors 1995, 37, 65–84. [Google Scholar] [CrossRef]
- De Winter, J.C.F.; Eisma, Y.B.; Cabrall, C.D.D.; Hancock, P.A.; Stanton, N.A. Situation Awareness Based on Eye Movements in Relation to the Task Environment. Cogn. Technol. Work 2019, 21, 99–111. [Google Scholar] [CrossRef]
- Endsley, M.R. The Divergence of Objective and Subjective Situation Awareness: A Meta-Analysis. J. Cogn. Eng. Decis. Mak. 2020, 14, 34–53. [Google Scholar] [CrossRef]
- Endsley, M.R. A Systematic Review and Meta-Analysis of Direct Objective Measures of Situation Awareness: A Comparison of SAGAT and SPAM. Hum. Factors 2021, 63, 124–150. [Google Scholar] [CrossRef]
- Dorton, S.L.; Maryeski, L.R.; Costello, R.P.; Abrecht, B.R. A Case for User-Centered Design in Satellite Command and Control. Aerospace 2021, 8, 303. [Google Scholar] [CrossRef]
- Loft, S.; Bowden, V.; Braithwaite, J.; Morrell, D.B.; Huf, S.; Durso, F.T. Situation Awareness Measures for Simulated Submarine Track Management. Hum. Factors 2015, 57, 298–310. [Google Scholar] [CrossRef]
- Charles, R.L.; Nixon, J. Measuring Mental Workload Using Physiological Measures: A Systematic Review. Appl. Ergon. 2019, 74, 221–232. [Google Scholar] [CrossRef]
- Van de Merwe, K.; van Dijk, H.; Zon, R. Eye Movements as an Indicator of Situation Awareness in a Flight Simulator Experimen. Int. J. Aviat. Psychol. 2012, 22, 78–95. [Google Scholar] [CrossRef]
- Catherwood, D.; Edgar, G.K.; Nikolla, D.; Alford, C.; Brookes, D.; Baker, S.; White, S. Mapping Brain Activity during Loss of Situation Awareness: An EEG Investigation of a Basis for Top-down Influence on Perception. Hum. Factors 2014, 56, 1428–1452. [Google Scholar] [CrossRef]
- Yeo, L.G.; Sun, H.; Liu, Y.; Trapsilawati, F.; Sourina, O.; Chen, C.-H.; Mueller-Wittig, W.; Ang, W.T. Mobile EEG-Based Situation Awareness Recognition for Air Traffic Controllers. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC) IEEE, Banff, AB, Canada, 5–8 October 2017; pp. 3030–3035. [Google Scholar]
- Wen, T.Y.; Aris, S.A.M. Electroencephalogram (EEG) Stress Analysis on Alpha/Beta Ratio and Theta/Beta Ratio. Indones. J. Electr. Eng. Comput. Sci. 2020, 17, 175. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Liang, N.; Pitts, B.J.; Prakah-Asante, K.O.; Curry, R.; Duerstock, B.S.; Wachs, J.P.; Yu, D. Physiological Measurements of Situation Awareness: A Systematic Review. Hum. Factors 2020, 1–22. [Google Scholar] [CrossRef]
- Cohen, M.X. Where Does EEG Come From and What Does It Mean? Trends Neurosci. 2017, 40, 208–218. [Google Scholar] [CrossRef]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F. Measuring Neurophysiological Signals in Aircraft Pilots and Car Drivers for the Assessment of Mental Workload, Fatigue and Drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef]
- Wanyan, X.; Zhuang, D.; Lin, Y.; Xiao, X.; Song, J.-W. Influence of Mental Workload on Detecting Information Varieties Revealed by Mismatch Negativity during Flight Simulation. Int. J. Ind. Ergon. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, M.; Liu, J.; Zheng, C. Electroencephalogram and Electrocardiograph Assessment of Mental Fatigue in a Driving Simulator. Accid. Anal. Prev. 2012, 45, 83–90. [Google Scholar] [CrossRef]
- Eoh, H.J.; Chung, M.K.; Kim, S.-H. Electroencephalographic Study of Drowsiness in Simulated Driving with Sleep Deprivation. Int. J. Ind. Ergon. 2005, 35, 307–320. [Google Scholar] [CrossRef]
- Kaur, A.; Chaujar, R.; Chinnadurai, V. Effects of Neural Mechanisms of Pretask Resting EEG Alpha Information on Situational Awareness: A Functional Connectivity Approach. Hum. Factors 2020, 62, 1150–1170. [Google Scholar] [CrossRef]
- Kästle, J.L.; Anvari, B.; Krol, J.; Wurdemann, H.A. Correlation between Situational Awareness and EEG Signals. Neurocomputing 2021, 432, 70–79. [Google Scholar] [CrossRef]
- Li, R.; Lan, Z.; Cui, J.; Sourina, O.; Wang, L. EEG-Based Recognition of Driver State Related to Situation Awareness Using Graph Convolutional Networks. In Proceedings of the 2020 International Conference on Cyberworlds (CW) IEEE, Caen, France, 29 September–1 October 2020. [Google Scholar]
- Feng, C.; Wanyan, X.; Yang, K.; Zhuang, D.; Wu, X. A Comprehensive Prediction and Evaluation Method of Pilot Workload. Technol. Health Care 2018, 26, 65–78. [Google Scholar] [CrossRef]
- Wei, Z.; Zhuang, D.; Wanyan, X.; Liu, C.; Zhuang, H. A Model for Discrimination and Prediction of Mental Workload of Aircraft Cockpit Display Interface. Chin. J. Aeronaut. 2014, 27, 1070–1077. [Google Scholar] [CrossRef]
- Santiago-Espada, Y.; Myer, R.R.; Latorella, K.A.; Comstock, J.R. The Multi-Attribute Task Battery II (MATB-II) Software for Human Performance and Workload Research: A User’s Guide; National Aeronautics and Space Administration, Langley Research Center: Hampton, VA, USA, 2011. [Google Scholar]
- Ferraro, J.C.; Mouloua, M. Effects of Automation Reliability on Error Detection and Attention to Auditory Stimuli in a Multi-Tasking Environment. Appl. Ergon. 2021, 91, 103303. [Google Scholar] [CrossRef]
- Liu, S.; Nam, C.S. Quantitative Modeling of User Performance in Multitasking Environments. Comput. Hum. Behav. 2018, 84, 130–140. [Google Scholar] [CrossRef]
- Wickens, C.D.; Gutzwiller, R.S.; Vieane, A.; Clegg, B.A.; Sebok, A.; Janes, J. Time Sharing between Robotics and Process Control: Validating a Model of Attention Switching. Hum. Factors 2016, 58, 322–343. [Google Scholar] [CrossRef]
- Butchibabu, A.; Sparano-Huiban, C.; Sonenberg, L.; Shah, J. Implicit Coordination Strategies for Effective Team Communication. Hum. Factors 2016, 58, 595–610. [Google Scholar] [CrossRef]
- Li, H.; Huang, G.; Lin, Q.; Zhao, J.; Fu, Q.; Li, L.; Mao, Y.; Wei, X.; Yang, W.; Wang, B.; et al. EEG Changes in Time and Time-Frequency Domain During Movement Preparation and Execution in Stroke Patients. Front. Neurosci. 2020, 14, 827. [Google Scholar] [CrossRef]
- Teplan, M. Fundamentals of EEG Measurement. Meas. Sci. Rev. 2002, 2, 11. [Google Scholar]
- Trapsilawati, F.; Herliansyah, M.K.; Nugraheni, A.S.A.N.S.; Fatikasari, M.P.; Tissamodie, G. EEG-Based Analysis of Air Traffic Conflict: Investigating Controllers’ Situation Awareness, Stress Level and Brain Activity during Conflict Resolution. J. Navig. 2020, 73, 678–696. [Google Scholar] [CrossRef]
- Fernandez Rojas, R.; Debie, E.; Fidock, J.; Barlow, M.; Kasmarik, K.; Anavatti, S.; Garratt, M.; Abbass, H. Electroencephalographic Workload Indicators during Teleoperation of an Unmanned Aerial Vehicle Shepherding a Swarm of Unmanned Ground Vehicles in Contested Environments. Front. Neurosci. 2020, 14, 40. [Google Scholar] [CrossRef]
- Armstrong, R.A. When to Use the Bonferroni Correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Armstrong, R.A. Recommendations for Analysis of Repeated-Measures Designs: Testing and Correcting for Sphericity and Use of MANOVA and Mixed Model Analysis. Ophthalmic Physiol. Opt. 2017, 37, 585–593. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.; Mishra, P.; Pandey, G. Application of Student’s t-Test, Analysis of Variance, and Covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef]
- De Giorgi, M.G.; Strafella, L.; Menga, N.; Ficarella, A. Intelligent Combined Neural Network and Kernel Principal Component Analysis Tool for Engine Health Monitoring Purposes. Aerospace 2022, 9, 118. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Li, P.; Li, H.; Liew, Y. A New Method for Remote Sensing Satellite Observation Effectiveness Evaluation. Aerospace 2022, 9, 317. [Google Scholar] [CrossRef]
- Frohlich, J.; Toker, D.; Monti, M.M. Consciousness among Delta Waves: A Paradox? Brain 2021, 144, 2257–2277. [Google Scholar] [CrossRef]
- Ren, M.; Xu, J.; Zhao, J.; Zhang, S.; Wang, W.; Xu, S.; Zhou, Z.; Chen, X.; Chen, S.; Li, Y.; et al. The Modulation of Working-Memory Performance Using Gamma-Electroacupuncture and Theta-Electroacupuncture in Healthy Adults. Evid. Based Complement. Alternat. Med. 2021, 2021, 2062718. [Google Scholar] [CrossRef]
- Frey, J.N.; Ruhnau, P.; Weisz, N. Not so Different after All: The Same Oscillatory Processes Support Different Types of Attention. Brain Res. 2015, 1626, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Khanna, P.; Carmena, J.M. Neural Oscillations: Beta Band Activity across Motor Networks. Curr. Opin. Neurobiol. 2015, 32, 60–67. [Google Scholar] [CrossRef]
- Spitzer, B.; Haegens, S. Beyond the Status Quo: A Role for Beta Oscillations in Endogenous Content (Re)Activation. eNeuro 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Shayesteh, S.; Jebelli, H. Enhanced Situational Awareness in Worker-Robot Interaction in Construction: Assessing the Role of Visual Cues. In Proceedings of the Construction Research Congress 2022, Arlington, VA, USA, 9–12 March 2022; pp. 422–430. [Google Scholar]
- Abbass, H.A.; Tang, J.; Amin, R.; Ellejmi, M.; Kirby, S. Augmented Cognition Using Real-Time EEG-Based Adaptive Strategies for Air Traffic Control. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Chicago, IL, USA, 27–31 October 2014; Volume 58, pp. 230–234. [Google Scholar]
- Jap, B.T.; Lal, S.; Fischer, P. Comparing Combinations of EEG Activity in Train Drivers during Monotonous Driving. Expert Syst. Appl. 2011, 38, 996–1003. [Google Scholar] [CrossRef]
- Angelidis, A.; van der Does, W.; Schakel, L.; Putman, P. Frontal EEG Theta/Beta Ratio as an Electrophysiological Marker for Attentional Control and Its Test-Retest Reliability. Biol. Psychol. 2016, 121, 49–52. [Google Scholar] [CrossRef]
- Wen, T.Y.; Bani, N.A.; Muhammad-Sukki, F.; Mohd Aris, S.A. Electroencephalogram (EEG) Human Stress Level Classification Based on Theta/Beta Ratio. Int. J. Integr. Eng. 2020, 12, 174–180. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R.; Cox, K.R.; Geuss, M.N.; Zaroukian, E.G.; Morris, K.M. The Validity of Situation Awareness for Performance: A Meta-Analysis. Theor. Issues Ergon. Sci. 2022, 23, 221–244. [Google Scholar] [CrossRef]







| Waves (μV2) | Region × Laterality × SA Group | Region × SA Group | Laterality × SA Group | SA Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | F | p | η2 | F | p | η2 | |
| δ | 0.879 | 0.437 | 0.038 | 0.155 | 0.751 | 0.007 | 0.974 | 0.378 | 0.042 | 3.020 | 0.096 | 0.121 |
| θ | 0.653 | 0.556 | 0.029 | 0.003 | 0.967 | <0.001 | 1.450 | 0.247 | 0.062 | 3.272 | 0.084 | 0.129 |
| α | 0.945 | 0.429 | 0.041 | 0.455 | 0.560 | 0.020 | 0.266 | 0.733 | 0.012 | 0.911 | 0.350 | 0.040 |
| β | 0.453 | 0.681 | 0.020 | 0.639 | 0.452 | 0.028 | 0.392 | 0.667 | 0.018 | 0.490 | 0.491 | 0.022 |
| Waves (%) | Region × Laterality × SA Group | Region × SA Group | Laterality × SA Group | SA Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | F | p | η2 | F | p | η2 | |
| δ | 1.460 | 0.234 | 0.062 | 0.239 | 0.694 | 0.011 | 1.854 | 0.171 | 0.078 | 0.871 | 0.361 | 0.038 |
| θ | 0.535 | 0.652 | 0.024 | 0.246 | 0.673 | 0.011 | 0.134 | 0.859 | 0.006 | 1.439 | 0.243 | 0.061 |
| α | 0.663 | 0.575 | 0.029 | 0.421 | 0.623 | 0.019 | 1.448 | 0.246 | 0.062 | 0.276 | 0.064 | 0.012 |
| β | 2.204 | 0.097 | 0.091 | 4.467 | 0.028 * | 0.169 | 1.918 | 0.160 | 0.080 | 6.911 | 0.015 * | 0.239 |
| SW/FW Ratios | Region × Laterality × SA Group | Region × SA Group | Laterality × SA Group | SA Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | η2 | F | p | η2 | F | p | η2 | F | p | η2 | |
| θ/β | 2.228 | 0.105 | 0.092 | 1.185 | 0.303 | 0.051 | 1.572 | 0.221 | 0.067 | 9.610 | 0.005 * | 0.304 |
| α/β | 3.404 | 0.020 * | 0.134 | 5.920 | 0.007 * | 0.212 | 1.057 | 0.343 | 0.046 | 4.040 | 0.057 | 0.155 |
| (θ + α)/β | 3.103 | 0.033 * | 0.124 | 2.778 | 0.092 | 0.112 | 1.770 | 0.184 | 0.074 | 9.072 | 0.006 * | 0.292 |
| (θ + α)/(α + β) | 1.180 | 0.321 | 0.051 | 0.299 | 0.661 | 0.013 | 0.708 | 0.485 | 0.031 | 5.761 | 0.025 * | 0.208 |
| Component | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | F1 | F2 | F3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode Sites | β Relative Power | θ/β | α/β | (θ + α)/β | (θ + α)/(α + β) | |||||||||||
| F3 | 0.788 | −0.065 | 0.492 | 0.762 | 0.419 | 0.441 | ||||||||||
| FZ | 0.841 | 0.171 | 0.449 | 0.863 | −0.074 | 0.446 | 0.687 | 0.558 | 0.338 | |||||||
| F4 | 0.790 | −0.120 | 0.518 | 0.721 | 0.406 | 0.402 | ||||||||||
| C3 | −0.910 | 0.138 | 0.074 | 0.939 | 0.002 | 0.083 | 0.890 | −0.315 | 0.109 | 0.843 | 0.485 | −0.004 | ||||
| CZ | −0.932 | −0.006 | 0.060 | 0.938 | 0.209 | 0.027 | 0.964 | −0.105 | 0.094 | 0.729 | 0.598 | −0.088 | ||||
| C4 | −0.899 | 0.145 | 0.073 | 0.970 | 0.009 | −0.001 | 0.899 | −0.357 | 0.051 | 0.835 | 0.484 | −0.064 | ||||
| P3 | −0.918 | 0.096 | 0.164 | 0.947 | −0.030 | −0.122 | 0.629 | −0.743 | 0.049 | 0.903 | −0.363 | −0.056 | 0.859 | 0.387 | −0.214 | |
| PZ | −0.881 | 0.053 | 0.267 | 0.916 | 0.120 | −0.338 | 0.673 | −0.692 | 0.017 | 0.923 | −0.264 | −0.210 | 0.738 | 0.497 | −0.416 | |
| P4 | −0.916 | 0.116 | 0.244 | 0.883 | 0.008 | −0.303 | 0.683 | −0.690 | 0.046 | 0.890 | −0.305 | −0.180 | 0.767 | 0.351 | −0.414 | |
| Validation Methods | SA Group | Predicted Accuracy (%) | Average Accuracy (%) | |
|---|---|---|---|---|
| HSA | LSA | |||
| Original validation | HSA | 83.3 | 16.7 | 83.3 |
| LSA | 16.7 | 83.3 | ||
| Cross-validation | HSA | 58.3 | 41.7 | 70.8 |
| LSA | 16.7 | 83.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Liu, S.; Wanyan, X.; Chen, H.; Min, Y.; Ma, Y. EEG Feature Analysis Related to Situation Awareness Assessment and Discrimination. Aerospace 2022, 9, 546. https://doi.org/10.3390/aerospace9100546
Feng C, Liu S, Wanyan X, Chen H, Min Y, Ma Y. EEG Feature Analysis Related to Situation Awareness Assessment and Discrimination. Aerospace. 2022; 9(10):546. https://doi.org/10.3390/aerospace9100546
Chicago/Turabian StyleFeng, Chuanyan, Shuang Liu, Xiaoru Wanyan, Hao Chen, Yuchen Min, and Yilan Ma. 2022. "EEG Feature Analysis Related to Situation Awareness Assessment and Discrimination" Aerospace 9, no. 10: 546. https://doi.org/10.3390/aerospace9100546